Then the stress must be oxidative or as result of the unsuccessful metabolization and/or increased LDH, AST, ALT, GGT, bilirubin, etc (enzymatic) due to some increased structural integrity of the molecule. This makes me even more confused as creating a derivative whether 17b-esterification as for injectables or 17a-alkylation for orals whether a methyl or ethyl group (potency) would be similarly hepatotoxic. But obviously they are not.
If a molecule is able to weather the inhospitable environment of the gut, I agree it would seem unlikely to be phased by any enzymatic activity in plasma (esterase, 4a and 5a-reductase, etc) or the liver yet obviously they are.
This would lead me to question why any AAS is administered orally at all. Or why orally administered steroids are any more hepatotoxic than injectable 17b-esterified compounds.
The reason I'm interested in this is not because of enjoyment but rather because I have not seen on any other board an in depth look at 1st pass effect, 2nd pass effect, exactly why one thing is more toxic from another and why different compounds function well together and why some dervitaves are more potent than others. I would like our members to have a basic understanding of this in layman's terms without wandering to far into the tall weeds with our collective egghead BS.
This would go some ways to reducing the ridiculous polypharmaceutical blasts I see on there that just make me cringe.
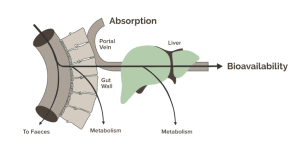
I'm leaving the graphic below so I know where to find when I (or perhaps we if you're interested) compile this information and make it more easily understandable.
View attachment 151619
Peter Bond and Bill Llewellyn wrote a paper on their proposed hypothesis for hepatotoxicity which I think explains things well. See Bond P, Llewellyn W, Van Mol P. Anabolic androgenic steroid-induced hepatotoxicity. Med Hypotheses. 2016 Aug;93:150-3. doi: 10.1016/j.mehy.2016.06.004. Epub 2016 Jun 5. PMID: 27372877.
Bond's Book on Steroids delves into the mechanisms and has illustrative graphics: worth a read.
Basically, from my notes on AAS-induced hepatotoxicity, the putative mechanisms are:
Hepatotoxicity = resistance to hepatic breakdown x potency to activate AR
There is a likely causal relationship between AAS and hepatoxicity via:
- AR activation ⇒ ⇑mitochondrial fatty acid β-oxidation (Bond citing 295, 397) [⇑CPT1 mRNA, rate-limiting enzyme in mitochondrial FA oxidation], 22Rv1 human prostate carcinoma epithelial cell line ⇒ ⇑ROS
- By corollary, fluoxymesterone and methylandrostanolone ⇒ ⇑rat liver CPT1 activity (Bond citing 192); rat hepatocyte mitochondrial swelling and reduced visual acuity of mitochondrial cristae (Bond citing 181):
-- ROS production causes mitochondrial swelling and subsequent apoptosis (Bond citing 80)
Markers
ASAT & ALAT: enzymes that occur in high concentrations in liver cells & leak into concentration when liver membrane & cell damage. However, non-specific. These enzymes are also present in muscle cells and ↑ by muscular work
GGT: enzyme present in cells from liver, kidney, pancreas & liver, mostly wherever biliary epithelial cells. ↑ most in hepatobiliary (liver, gall bladder, bile duct) disease. A sensitive marker of cholestasic liver disorders. Somewhat nonspecific.
ALP: most pronounced elevation in cholestasis, but smaller increases in all sorts of liver disorders.
Taken together, these markers suggest liver damage & possible bile duct obstruction.
And from an article sourced from
**broken link removed**
Date: Monday, July 18, 2011
A few words on the hepatotoxicity of 17a-methylated androgens/anabolics
1. 17a-methylated androgens/anabolics are hepatotoxic.
The liver toxicity of steroids is an under-researched field, but there seems to be a strong correlation between how easily the body can metabolize a steroid & its toxicity. Metribolone -- a truly excessively toxic compound -- is often referred to in the literature as a 'non-metabolizable androgen'. (1, 2, 3, etc.) Mibolerone, another deadly-toxic anabolic steroid, is also effectively 'non-metabolizable': The main metabolite of mibolerone in humans is... unchanged mibolerone. And by a very wide margin.
Methylstenbolone, which is resistant to 17b-HSD and 3b-HSD, is obviously difficult for the body to clear. It should therefore be no safer, no less toxic, than Superdrol or M1T -- compounds which share very similar traits.
2. Liver injury due to oral anabolic use typically manifests itself as cholestasis.
Hepatotoxicity induced by oral anabolic compounds tends to be characterized by enlargement of periportal hepatocytes, impairment of bile flow & dramatically increased serum levels of AST, ALT and GGT. In other words, cholestasis... but let's examine this a little bit further.
The word "cholestasis" gets thrown around a lot, but it can mean two very different things: The physical obstruction of hepatic bile flow -or- the impairment of bile secretion. In the former case, there is a mechanical block in the bile duct system; in the latter, bile is held in hepatocytes or cholangiocytes as it cannot be secreted. In both cases, what happens thereafter is that the retained hydrophobic bile salts -- which are strongly cytotoxic -- lead to cellular injury, then apoptosis, then necrosis, often followed by an inflammatory reaction and tissue fibrosis. This tissue damage, if advanced enough, can physically destroy bile ducts, worsening the condition.
The obstruction of bile flow is typically not something you'd experience after exposure to any toxin; it is the almost exclusive domain of inherited or autoimmune diseases which leave fibrotic lesions or scar-tissue in the liver, such as cystic fibrosis, primary biliary cirrhosis, and so on. Exposure to oral anabolic compounds can, however, result in the second form of cholestasis -- bile retention in hepatocytes -- thus the enlarged hepatocytes observed after their use.
3. There are three fundamental ways of preventing/treating cholestasis:
1. Metabolic induction of hydrophobic bile acid detoxification
2. Stimulation of impaired bile secretion
3. Protection of hepatocytes from the toxic effects of hydrophobic bile acids and/or inhibition of hepatocyte apoptosis.
Cholestatic liver damage is caused by bile acid accumulation... But not all bile acids are toxic. Generally speaking, the fewer hydroxyl groups they bear, the more hydrophobic and cytotoxic they are. Hence lithocholic acid is markedly cytotoxic, deoxycholic acid is very slightly cytotoxic, and cholic acid is essentially non-cytotoxic. Treatment #1 would involve hastening the metabolic conversion of the more toxic bile acids to hydrophilic, less toxic compounds --- or increasing the synthesis of hydrophilic bile acids from cholesterol, which would decrease the cytotoxicity of the entire bile pool as a whole. This can seemingly be achieved with the oral administration of ursodeoxycholic acid (UDCA), which has been reported to activate the PXR/SXR nuclear receptor in hepatocytes, which then activates bile acid–metabolizing enzymes. It is reasonable to assume that Tauroursodeoxycholic acid (TUDCA), the taurine conjugate of UDCA, should have the same effect.
As for #2... Bile secretion at the level of the hepatocyte is carried out by a group of transporter proteins: The bile salt export pump (BSEP), the phospholipid export pump (MDR3), the canalicular bilirubin conjugate export pump (MRP2), and a chloride-bicarbonate anion exchanger (AE2) for bicarbonate excretion. BSEP is the driving factor behind bile-acid dependent secretion, and MRP2/AE2 are the major forces behind bile-acid-independent bile secretion. Hydrophilic bile acids such as UDCA & TUDCA (and even, partially, cholic acid) have been shown to increase expression of BSEP mRNA; they activate BSEP coactivators by binding to the Farnesoid X Receptor (the "bile acid receptor"); they phosphorylate the BSEP protein via a Ca+/PKCa-mediated mechanism; lastly, they stimulate Cl -/HCO3 - exchange via this same PKCa induction, thus increasing AE2 levels.
Taken together, the above effects drastically enhance secretion of potentially toxic bile acids.
...#3 can be complicated, but I will explain briefly: Certain toxic bile salts activate the Fas Death Receptor on hepatocytes. This leads to a cascade of dozens of protein interactions & ultimately to cell death. TUDCA, UDCA, and certain other compounds can diminish Fas–induced apoptosis, but, as far as I am aware, the exact mechanism is not known at this time. Fas activation here is not ligand-dependent, so the 'obvious' mechanism is out the window. The mechanism could, however, involve activation of the EGFR, which activates MAPK & the MAPK-mediated 'survival pathway' in hepatocytes; it might also involve inhibition, somewhere along the line, of the proapoptotic proteins Bax and Bid.


































.gif)