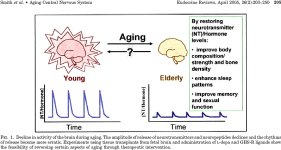
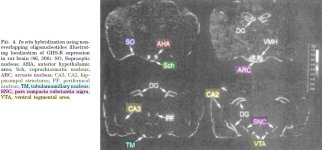
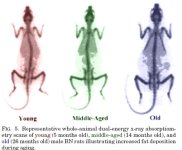
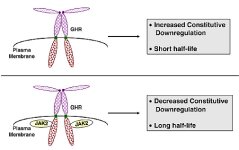
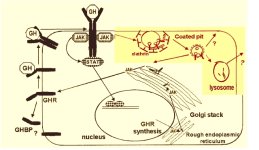
REFERENCES:
[1] - [11] - UNUSED
[12] J.F. Bazan, Structural design and molecular evolution of a cytokine receptor superfamily, Proc. Natl. Acad. Sci. U. S. A. 87 (1990) 6934–6938.
[13] D.W. Leung, S.A. Spencer, G. Cachianes, R.G. Hammonds, C. Collins,W.J. Henzel, R. Barnard, M.J. Waters, W.I. Wood, Growth hormone receptor and serum binding protein: purification, cloning and expression, Nature 330 (1987) 537–543.
[14] S.J. Frank, J.L. Messina, Growth hormone receptor, in: J.J. Oppenheim, M. Feldman (Eds.), Cytokine Reference On-Line, Academic Press, Harcourt, London, UK, 2002, pp. 1–21, Website,
www.academicpress.com/cytokinereference, 24-hour free access.
[15] C. Carter Su, J. Schwartz, L.S. Smit, Molecular mechanism of growth hormone action, Annu. Rev. Physiol. 58 (1996) 187–207.
[16] S.J. Frank, J.J. O'Shea (Eds.), Recent Advances in Cytokine Signal Transduction: Lessons from Growth Hormone and other Cytokines, 1999, Greenwich, CT.
[17] Brown, R. J., Adams, J. J., Pelekanos, R. A.,Wan,Y., McKinstry,W. J., Palethorpe, K., et al. (2005). Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat. Struct. Mol. Biol., 12(9), 814–821.
[18] Ihle, J. N., & Gilliland, D. G. (2007). Jak2: Normal function and role in hematopoietic disorders. Curr. Opin. Genet. Dev., 17(1), 8–14.
[19] Waters, M. J., Hoang, H. N., Fairlie, D. P., Pelekanos, R. A., & Brown, R. J. (2006). New insights into growth hormone action. J. Mol. Endocrinol., 36(1), 1–7.
[20] Zhu, T., Ling, L., & Lobie, P. E. (2002). Identification of a JAK2- independent pathway regulating growth hormone (GH)-stimulated p44/42 mitogen-activated protein kinase activity. GH activation of Ral and phospholipaseDis Src-dependent. J. Biol. Chem., 277(47), 45592–45603.
[21] Rowlinson, S. W., Yoshizato, H., Brown, R. J., Behncken, S. N., Barclay, J. L., Brooks, A. J., et al. An activation-related conformational change determines signalling pathway choice for the growth hormone (GH) receptor. Manuscript submitted for publication.
[22] Fresno Vara, J. A., Caceres, M. A., Silva, A., & Martin-Perez, J. (2001). Src family kinases are required for prolactin induction of cell proliferation. Mol. Biol. Cell, 12(7), 2171–2183.
[23] S.J. Frank, J.L. Messina, Growth hormone receptor, in: J.J. Oppenheim, M. Feldman (Eds.), Cytokine Reference On-Line, Academic Press, Harcourt, London, UK, 2002, pp. 1–21, Website,
www.academicpress.com/cytokinereference, 24-hour free access.
[24] C. Carter Su, J. Schwartz, L.S. Smit, Molecular mechanism of growth hormone action, Annu. Rev. Physiol. 58 (1996) 187–207.
[25] S.J. Frank, J.J. O'Shea (Eds.), Recent Advances in Cytokine Signal Transduction: Lessons from Growth Hormone and other Cytokines, 1999, Greenwich, CT.
[26] L.H. Hansen, X. Wang, J.J. Kopchick, P. Bouchelouche, J.H. Nielsen, E.D. Galsgaard, N. Billestrup, Identification of tyrosine residues in the intracellular domain of the growth hormone receptor required for transcriptional signaling and Stat5 activation, J. Biol. Chem. 271 (1996) 12669–12673.
[27] L.S. Smit, D.J. Meyer, N. Billestrup, G. Norstedt, J. Schwartz, C. Carter-Su, The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH, Mol. Endocrinol. 10 (1996) 519–533.
[28] A. Sotiropoulos, S. Moutoussamy, F. Renaudie, M. Clauss, C. Kayser, F. Gouilleux, P.A. Kelly, J. Finidori, Differential activation of Stat3 and Stat5 by distinct regions of the growth hormone receptor, Mol. Endocrinol. 10 (1996) 998–1009.
[29] X. Wang, C.J. Darus, B.C. Xu, J.J. Kopchick, Identification of growth hormone receptor (GHR) tyrosine residues required for GHR phosphorylation and JAK2 and STAT5 activation, Mol. Endocrinol. 10 (1996) 1249–1260.
[30] W. Yi, S.O. Kim, J. Jiang, S.H. Park, A.S. Kraft, D.J. Waxman, S.J. Frank, Growth hormone receptor cytoplasmic domain differentially promotes tyrosine phosphorylation of signal transducers and activators of transcription 5b and 3 by activated JAK2 kinase, Mol. Endocrinol. 10 (1996) 1425–1443.
[31] P.L. Bergad, H.M. Shih, H.C. Towle, S.J. Schwarzenberg, S.A. Berry, Growth hormone induction of hepatic serine protease inhibitor 2.1 transcription is mediated by a Stat5-related factor binding synergistically to two alphaactivated sites, J. Biol. Chem. 270 (1995) 24903–24910.
[32] H.W. Davey, M.J. McLachlan, R.J. Wilkins, D.J. Hilton, T.E. Adams, STAT5b mediates the GH-induced expression of SOCS-2 and SOCS-3 mRNA in the liver, Mol. Cell Endocrinol. 158 (1999) 111–116.
[33] H.W. Davey, T. Xie, M.J. McLachlan, R.J. Wilkins, D.J. Waxman, D.R. Grattan, STAT5b is required for GH-induced liver IGF-I gene expression, Endocrinology 142 (2001) 3836–3841.
[34] G.B. Udy, R.P. Towers, R.G. Snell, R.J. Wilkins, S.H. Park, P.A. Ram, D.J. Waxman, H.W. Davey, Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression, Proc. Natl. Acad. Sci. U. S. A. 94 (1997) 7239–7244.
[35] G.T. Ooi, K.R. Hurst, M.N. Poy, M.M. Rechler, Y.R. Boisclair, Binding of STAT5a and STAT5b to a single element resembling a alpha-interferon-activated sequence mediates the growth hormone induction of the mouse acid-labile subunit promoter in liver cells, Molecular. Endocrinology. 12 (1998) 675–687.
[36] J.Woelfle, J. Billiard, P. Rotwein, Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b, J. Biol. Chem. 278 (2003) 22696–22702
[37] A. Sotiropoulos, M. Perrot-Applanat, H. Dinerstein, A. Pallier, M.C. Postel-Vinay, J. Finidori, P.A. Kelly, Distinct cytoplasmic regions of the growth hormone receptor are required for activation of JAK2, mitogen-activated protein kinase, and transcription, Endocrinology 135 (1994) 1292–1298.
[38] J.A. Vanderkuur, X. Wang, L. Zhang, G.S. Campbell, G. Allevato, N. Billestrup, G. Norstedt, C. Carter-Su, Domains of the growth hormone receptor required for association and activation of JAK2 tyrosine kinase, J. Biol. Chem. 269 (1994) 21709–21717.
[39] C. Moller, A. Hansson, B. Enberg, P.E. Lobie, G. Norstedt, Growth hormone (GH) induction of tyrosine phosphorylation and activation of mitogen-activated protein kinases in cells transfected with rat GH receptor cDNA, J. Biol. Chem. 267 (1992) 23403–23408.
[40] L.S. Argetsinger, G.W. Hsu, M.G.J. Myers, N. Billestrup, M.F. White, C. Carter-Su, Growth hormone, interferon-alpha, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1, J. Biol. Chem. 270 (1995) 14685–14692.
[41] L.S. Argetsinger, G. Norstedt, N. Billestrup, M.F. White, C. Carter-Su, Growth hormone, interferon-alpha, and leukemia inhibitory factor utilize insulin receptor substrate-2 in intracellular signaling, J. Biol. Chem. 271 (1996) 29415–29421
[42] C. Hodge, J. Liao, M. Stofega, K. Guan, C. Carter-Su, J. Schwartz, Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2, J. Biol. Chem. 273 (1998) 31327–31336.
[43] L. Liang, T. Zhou, J. Jiang, J.H. Pierce, T.A. Gustafson, S.J. Frank, Insulin receptor substrate-1 enhances growth hormone-induced proliferation, Endocrinology 140 (1999) 1972–1983.
[44] S.O. Kim, J.C. Houtman, J. Jiang, J.M. Ruppert, P.J. Bertics, S.J. Frank, Growth hormone-induced alteration in ErbB-2 phosphorylation status in 3T3-F442A fibroblasts, J. Biol. Chem. 274 (1999) 36015–36024.
[45] Y. Huang, Y. Chang, X.Wang, J. Jiang, S.J. Frank, Growth hormone alters epidermal growth factor receptor binding affinity via activation of ERKs in 3T3-F442A cells, Endocrinology 145 (2004) 3297–3306
[46] J.A. Costoya, J. Finidori, S. Moutoussamy, R. Searis, J. Devesa, V.M. Arce, Activation of growth hormone receptor delivers an antiapoptotic signal: evidence for a role of Akt in this pathway, Endocrinology 140 (1999) 5937–5943.
[47] S. Jeay, G.E. Sonenshein, P.A. Kelly, M.C. Postel-Vinay, E. Baixeras, Growth hormone exerts antiapoptotic and proliferative effects through two different pathways involving nuclear factor-kappaB and phosphatidylinositol 3-kinase, Endocrinology 142 (2001) 147–156.
[48] E. Kilgour, I. Gout, N.G. Anderson, Requirement for phosphoinositide 3-OH kinase in growth hormone signalling to the mitogen-activated protein kinase and p70s6k pathways, Biochem. J. 315 (1996) 517–522.
[49] S.J. MacKenzie, S.J. Yarwood, A.H. Peden, G.B. Bolger, R.G. Vernon, M.D. Houslay, Stimulation of p70S6 kinase via a growth hormone-controlled phosphatidylinositol 3-kinase pathway leads to the activation of a PDE4A cyclic AMP-specific phosphodiesterase in 3T3-F442A preadipocytes, Proc. Natl. Acad. Sci. U. S. A. 95 (1998) 3549–3554.
[50] L. Liang, J. Jiang, S.J. Frank, Insulin receptor substrate-1-mediated enhancement of growth hormone-induced mitogen-activated protein kinase activation, Endocrinology 141 (2000) 3328–3336.
[51] G. Schwartzbauer, R.K. Menon, Regulation of growth hormone receptor gene expression, Mol. Genet. Metab. 63 (1998) 243–253.
[52] F. Talamantes, R. Ortiz, Structure and regulation of expression of the mouse GH receptor, J. Endocrinol. 175 (2002) 55–59.
[53] J. Gent, P. van Kerkhof, M. Roza, G. Bu, G.J. Strous, Ligand-independent growth hormone receptor dimerization occurs in the endoplasmic reticulum and is required for ubiquitin system-dependent endocytosis, Proc. Natl. Acad. Sci. U. S. A. 99 (2002) 9858–9863.
[54] - [58] - UNUSED
[59] K. He, X.Wang, J. Jiang, R. Guan, K.E. Bernstein, P.P. Sayeski, S.J. Frank, Janus kinase 2 determinants for growth hormone receptor association, surface assembly, and signaling, Mol. Endocrinol. 17 (2003) 2211–2227.
[60] R.J. Brown, J.J. Adams, R.A. Pelekanos, Y.Wan,W.J. McKinstry, K. Palethorpe, R.M. Seeber, T.A. Monks, K.A. Eidne, M.W. Parker, M.J. Waters, Model for growth hormone receptor activation based on subunit rotation within a receptor dimer, Nat. Struct. Mol. Biol. 12 (2005) 814–821.
[61] M.J. van den Eijnden, L.L. Lahaye, G.J. Strous, Disulfide bonds determine growth hormone receptor folding, dimerisation and ligand binding, J. Cell. Sci. 119 (2006) 3078–3086.
[62] K. He, K. Loesch, J.W. Cowan, X. Li, L. Deng, X. Wang, J. Jiang, S.J. Frank, JAK2 enhances the stability of the mature GH receptor. Endocrinology 145 (2005) 4755–4765.
[63] K. Loesch, L. Deng, X. Wang, K. He, J. Jiang, S.J. Frank, Endoplasmic reticulumassociated degradation of growth hormone receptor in Janus kinase 2-deficient cells, Endocrinology 148 (2007) 5955–5965
[64] - UNUSED
[65] X. Wang, K. He, M. Gerhart, Y. Huang, J. Jiang, R.J. Paxton, S. Yang, C. Lu, R.K. Menon, R.A. Black, G. Baumann, S.J. Frank, Metalloprotease-mediated GH receptor proteolysis and GHBP shedding. Determination of extracellular domain stem region cleavage site, J. Biol. Chem. 277 (2002) 50510–50519.
[66] K. Loesch, L. Deng, J.W. Cowan, X.Wang, K. He, J. Jiang, R.A. Black, S.J. Frank, JAK2 influences growth hormone receptor metalloproteolysis, Endocrinology 147 (2006) 2839–2849.
[67] K. Romisch, Endoplasmic reticulum-associated degradation, Annu. Rev. Cell. Dev. Biol. 21 (2005) 435–456.
[68] A. Ahner, J.L. Brodsky, Checkpoints in ER-associated degradation: excuse me, which way to the proteasome? Trends. Cell. Biol. 14 (2004) 474–478.
[69] A. Schmitz, V. Herzog, Endoplasmic reticulum-associated degradation: exceptions to the rule, Eur. J. Cell. Biol. 83 (2004) 501–509.
[70] - [79] - UNUSED
[80] J. Alele, J. Jiang, J.F. Goldsmith, X. Yang, H.G. Maheshwari, R.A. Black, G. Baumann, S.J. Frank, Blockade of growth hormone receptor shedding by a metalloprotease inhibitor, Endocrinology 139 (1998) 1927–1935.
[81] X. Wang, K. He, M. Gerhart, J. Jiang, R.J. Paxton, R.K. Menon, R.A. Black, G. Baumann, S.J. Frank, Reduced proteolysis of rabbit growth hormone (GH) receptor substituted with mouse GH receptor cleavage site, Mol. Endocrinol. 17 (2003) 1931–1943.
[82] G. Baumann, Growth hormone binding protein 2001, J. Pediatr. Endocrinol. Metab. 14 (2001) 355–375.
[83] G. Baumann, S.J. Frank, Metalloproteinases and the modulation of GH signaling, J. Endocrinol. 174 (2002) 361–368.
[84] R. Guan, Y. Zhang, J. Jiang, C.A. Baumann, R.A. Black, G. Baumann, S.J. Frank, Phorbol ester- and growth factor-induced growth hormone (GH) receptor proteolysis and GH-binding protein shedding: relationship to GH receptor downregulation, Endocrinology 142 (2001) 1137–1147.
[85] Y. Zhang, R. Guan, J. Jiang, J.J. Kopchick, R.A. Black, G. Baumann, S.J. Frank, Growth hormone (GH)-induced dimerization inhibits phorbol ester-stimulated GH receptor proteolysis, J. Biol. Chem. 276 (2001) 24565–24573.
[86] J. Jiang, X. Wang, K. He, X. Li, C. Chen, P.P. Sayeski, M.J. Waters, S.J. Frank, A Conformationally-sensitive GHR (Growth Hormone (GH) Receptor) antibody: impact on GH signaling and GHR proteolysis. Mol. Endocrinol. 18 (2004) 2981–2996.
[87] J.W. Cowan, X. Wang, R. Guan, K. He, J. Jiang, G. Baumann, R.A. Black, M.S. Wolfe, S.J. Frank, Growth hormone receptor is a target for presenilin-dependent gamma-secretase cleavage, J. Biol. Chem. 280 (2005) 19331–19342.
[88] - [89] - UNUSED
[90] M.S. Wolfe, R. Kopan, Intramembrane proteolysis: theme and variations, Science 305 (2004) 1119–1123.
[91] G.J. Strous, P. van Kerkhof, The ubiquitin–proteasome pathway and the regulation of growth hormone receptor availability, Mol. Cell. Endocrinol. 197 (2002) 143–151
[92] - [97] - UNUSED
[98] P. Roupas, A.C. Herington, Intracellular processing of growth hormone receptors by adipocytes in primary culture, Mol. Cell. Endocrinol. 57 (1988) 93–99.
[99] M.A. Lesniak, J. Roth, Regulation of receptor concentration by homologous hormone. Effect of human growth hormone on its receptor in IM-9 lymphocytes, J. Biol. Chem. 251 (1976) 3720–3729.
[100] N. Hizuka, P. Gorden, M.A. Lesniak, E. Van Obberghen, J.L. Carpentier, L. Orci, Polypeptide hormone degradation and receptor regulation are coupled to ligand internalization. A direct biochemical and morphologic demonstration, J. Biol. Chem. 256 (1981) 4591–4597.
[101] - [106] - UNUSED
[107] S. Moutoussamy, F. Renaudie, F. Lago, P.A. Kelly, J. Finidori, Grb10 identified as a potential regulator of growth hormone (GH) signaling by cloning of GH receptor target proteins, J. Biol. Chem. 273 (1998) 15906–15912.
[108] M.R. Stofega, J. Herrington, N. Billestrup, C. Carter-Su, Mutation of the SHP-2 binding site in growth hormone (GH) receptor prolongs GH-promoted tyrosyl phosphorylation of GH receptor, JAK2, and STAT5B, Mol. Endocrinol. 14 (2000) 1338–1350.
[109] P.A. Ram, D.J. Waxman, SOCS/CIS protein inhibition of growth hormonestimulated STAT5 signaling by multiple mechanisms, J. Biol. Chem. 274 (1999) 35553–35561.
[110] L. Du, G.P. Frick, L.R. Tai, A. Yoshimura, H.M. Goodman, Interaction of the growth hormone receptor with cytokine-induced Src homology domain 2 protein in rat adipocytes, Endocrinology 144 (2003) 868–876.
[111] S.O. Kim, J. Jiang, W. Yi, G.S. Feng, S.J. Frank, Involvement of the Src homology 2- containing tyrosine phosphatase SHP-2 in growth hormone signaling, J. Biol. Chem. 273 (1998) 2344–2354.
[112] J.G. Zhang, A. Farley, S.E. Nicholson, T.A. Willson, L.M. Zugaro, R.J. Simpson, R.L. Moritz, D. Cary, R. Richardson, G. Hausmann, B.J. Kile, S.B. Kent,W.S. Alexander, D. Metcalf, D.J. Hilton, N.A. Nicola, M. Baca, The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation, Proc. Natl. Acad. Sci. U. S. A. 96 (1999) 2071–2076.
[113] T. Landsman, D.J.Waxman, Role of the cytokine-induced SH2 domain-containing protein CIS in growth hormone receptor internalization, J. Biol. Chem. 280 (2005) 37471–37480.
[114] W. Yi, S.O. Kim, J. Jiang, S.H. Park, A.S. Kraft, D.J. Waxman, S.J. Frank, Growth hormone receptor cytoplasmic domain differentially promotes tyrosine phosphorylation of signal transducers and activators of transcription 5b and 3 by activated JAK2 kinase, Mol. Endocrinol. 10 (1996) 1425–1443.