Serum IGF-I levels and IGF-I gene splicing in muscle of healthy young males receiving rhGH Growth Hormone & IGF Research, In Press, Corrected Proof, Available online 16 September 2008, Michael Aperghis, Growth Hormone & IGF Research xxx (2008) xxx–xxx
Introduction
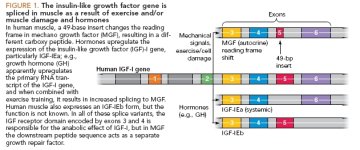
IGF-I is a ubiquitous peptide that is involved in a number of important functions during growth and development. It has been shown to have anabolic actions on muscle both in vivo and in vitro [1–3]. The IGF-I gene comprises six exons and has two promoter regions. The mature IGF-I peptide found in the circulation is encoded mainly by exons 3 and 4, whilst exons 5 and 6 encode most of the C-terminal E domain. This and the N-terminal sequences encoded by exons 1 or 2 are cleaved during maturation.
The IGF-I gene can be spliced both at the 50 and 30 ends. At the 50 end Class 1 or Class 2 IGF-I transcripts result depending upon their initiation from either promoter 1 (adjacent to exon 1) or 2 (adjacent to exon 2), respectively [4–6].
At the 30 end alternative splicing may result in three E domain peptides IGF-IEa, IGF-IEb and IGF-I Ec (MGF) [7– 9]. Thus a
total of at least six different splice variants of IGF-I is possible. It is now becoming clear that the 30 splice variants or E peptides may have distinct functions in growth and adaptation of skeletal muscle [10–12], but the role of the Class 1 and 2 transcripts is unknown.
IGF-I expression is regulated, in many tissues by growth hormone (GH) [13], and
GH administration results in increased circulating IGF-I peptide in animals as well as humans. In rats and sheep,
GH may preferentially upregulate Class 2 transcripts in the liver, the organ chiefly responsible for the production of serum IGF-I [14– 16]. The effects of GH on muscle 30 splice variant expression have been studied in elderly human subjects [17]. Recombinant hGH administration was shown to preferentially upregulate IGF-IEa mRNA, with MGF being increased when rhGH was combined with overloading exercise, but neither Class 1 nor Class 2 splice variants were measured in this study. Elderly individuals are a somewhat special case in that they have low levels of circulating GH. Indeed, the time course of IGF-IEa and MGF mRNA upregulation in muscle following a bolus injection of GH differs in GH deficient (lit/lit) andGH sufficient mice [18]. Again, Class 1 and 2 transcripts were not measured. Thus, little is known about the regulation of muscle 50 and 30 splice variant expression under conditions of normal GH status.
It is important to understand the role of GH on muscle IGF-I gene expression in healthy, young subjects not least because many athletes and bodybuilders use rhGH in an attempt to increase muscle bulk [19].
The aim of the present study was therefore to examine the effects of exogenous rhGH with and without acute resistance exercise on the expression of the 50 and 30 splice variants in the muscles of healthy young men.
...
Design: The study was a randomised double blind trial with a crossover design. Seven subjects were randomly assigned to a group receiving daily injections of rhGH (0.075 IU kg/ day) or placebo for a two week period. Following a one month washout, the groups were reversed.
Discussion
The main finding of the present study was that two weeks of rhGH administration resulted in a significant increase in circulating IGF-I but did not affect the expression in skeletal muscle of Class 1, 2 or 30 IGF-I transcripts. The data show for the first time that Class 1 and 2 transcripts are expressed in human skeletal muscle, and
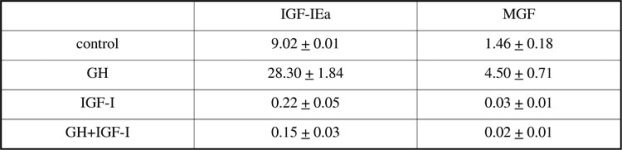
confirm our previous observation that IGF-IEa expression is two orders of magnitude higher than MGF expression. These two splice variants are known to have different physiological roles in murine [10] and human muscle progenitor cells [12]. As mentioned above, a direct comparison of Class 1 and 2 transcripts with IGF-IEa and MGF was not possible because different primers were used for the RT reactions. Nevertheless, the efficiency of reverse transcription reactions primed with random hexamers is expected to be lower than that of reactions primed with gene specific primers as used for IGF-IEa and MGF transcripts. Thus it is possible that reverse transcription or amplification of 30 products is less efficient than that of 50 products. This remains to be conclusively established for IGF-I transcripts.
It has been
reported that Class 2 IGF-I transcripts have greater stability than Class 1, whilst Class 1 transcripts in turn have a higher transcriptional/translational throughput [5]. Thus it might be expected that Class 1 transcripts respond more rapidly to changing hormonal profile. On the other hand it has been shown that Class 2 transcripts are preferentially upregulated by GH (at least in the liver), leading to the suggestion that it is the peptides translated from these transcripts that are secreted into the circulation [14,16]. In contrast, Class 1 transcripts would remain localized to the site of synthesis and have autocrine/paracrine actions [2,16,20–22]. Although transgenic mice overexpressing Class 1 IGF-IEa under control of muscle specific regulatory elements show increased IGF-I protein only in muscle [2,23], this may be the result of the promoter rather than the exon 1 encoded peptide. Over expression of Class 1 constructs in pigs and cultured muscle cells results in elevated IGF-I levels in blood plasma and culture medium, respectively [2,24], demonstrating that Class 1 peptides can be secreted. Thus, further studies are needed to clarify the role of the peptides encoded by exons 1 and 2.
The lack of effect of GH on IGF-I 30 transcript levels in muscle was somewhat unexpected given our previous observation of a 250% increase in muscle IGF-IEa mRNA in elderly subjects following five weeks rhGH treatment [17]. Subject numbers per treatment group were comparable between this study (n = 7) and the study performed on elderly individuals (n = 6 or 7), however the total number of subjects in the present study was lower as the cross over design was used in order to minimize intersubject variability.
We have previously reported that values of IGF-IEa and MGF transcripts can vary by 4-fold between subjects [9]. To obviate this effect we also examined data normalised to each baseline sample (placebo, pre exercise) and observed no effect.
However, the mean value of MGF expression in the GH + exercise group was higher than the remaining treatment groups. It is thus possible but unlikely, considering the marked increase in circulating IGF-I with GH administration, that the limited subject number and the low sensitivity of QPCR for low abundance transcripts may have masked an effect of GH in this group. A more
likely explanation for the observed lack of upregulation of IGF-I splice variants in skeletal muscle by GH i
s discussed.
The young subjects used herein were not GH deficient. Age is associated with a progressive decline in circulating levels of GH and IGF-I such that elderly individuals may be considered to be GH deficient [25]. In the study by Hameed and coworkers [17] rhGH administration to the elderly subjects (0.036 IU kg day) caused circulating IGF-I levels to rise by 70% from 20 nmol/l to 32 nmol/l, which is equivalent to the baseline levels measured in our young subjects. The increase in circulating IGF-I in our young subjects following treatment with rhGH (0.075 IU kg day) was more dramatic (180%, Fig. 1) than that observed in elderly subjects. A more robust effect of GH treatment might likewise be expected in muscles of young subjects.
The lack of IGF-I upregulation in observed thus suggest that GH dependent primary IGF-I gene transcription is already maximal in young men and not a limiting factor regulating splice variant expression in muscle. Evidence for differential regulation of local (muscle) IGF-I expression in GH normal and deficient states comes from a study in growth hormone- deficient mice [18]. When a bolus of GH was given to GH deficient (lit/lit) animals, both MGF and IGF-IEa isoforms were upregulated 12 h after the injection. In GH sufficient, lit/+ animals, although upregulation of both isoforms was initially observed 4 h after treatment, they did not remain elevated at 12 h. Thus, it would seem that although administration of GH results in transient upregulation of IGF-I 30 splice variants in mice with normal GH endocrinology, more persistent changes may occur in GH deficient animals. The findings presented herein are in accordance with these observations.
It might be argued that the dose or dosing period of GH used in the present study was insufficient to elicit a transcriptional response in the muscles of young men. Indeed, the doses reportedly used by body builders and weightlifters may be more than two fold higher than used here [19]. In the study showing upregulation of IGF-I in elderly human muscle, rhGH was administered for five rather than two weeks as reported herein [17].
However, a single bolus administration of GH has been shown to activate GH signalling pathways in human muscle [26] suggesting that in young individuals, muscle is GH sensitive.
The dose and administration period were nevertheless sufficient to elicit a major response from the liver, as evidenced by the 180% increase in serum IGF-I (Fig. 1).
The data presented here suggests that liver and skeletal muscle have different sensitivities to exogenous GH. Although most data regarding the tissue specific effects of GH administration has been obtained in GH deficient animal models, some experiments have been performed in GH sufficient animals. In sheep, ten weeks GH treatment led to increased IGF-I expression in liver but not muscle [27], whereas tissue injection of GH in male pigs resulted in increased expression of IGF-I mRNA in liver, adipose tissue, and in the semitendinosus muscle, but not in the longissimus muscle [28]. Furthermore, injection of a plasmid encoding GH in the tibialis anterior of young, male mice showed upregulation of IGF-IEa mRNA in the liver but not in the injected muscle [29]. The reason for this differential regulation is not known since no differences between liver and muscle transcripts have been reported. It has been shown, however, that the liver expresses tenfold more GH binding activity than muscle [30]
A system of negative feedback regulates the GH/ IGF-I axis. Interestingly, when C2C12 muscle cells are treated in culture with rhGH they increase both IGF-I Ea and MGF 30 splice variants but treatment with IGF-I alone and in combination with GH has an inhibitory effect and the cells are prevented from expressing either splice variant [31]. It is possible that a similar mechanism of negative feedback is operating on muscle in vivo. See:
Post #161 below
In the present study we did not observe the significant increases (2%–800%) in MGF mRNA expression following a one off resistance exercise bout as previously reported [9]. The most likely explanation for this is that the decreased sensitivity of QPCR for transcripts expressed at low levels (Fig. 2) masked any changes in expression. In addition, the 2.5 h time point may not be optimal for observing robust MGF upregulation, and studies in humans now measure MGF at least 24 h after an exercise bout [32]. In mice, the peak MGF response is observed 24 h after muscle damage [33].
The diverse role of MGF as a repair molecule is becoming increasingly clear [12,34–36]. Unaccustomed exercise results in muscle damage and soreness, and in the extreme case of child birth, this damage is associated with strikingly high levels of MGF mRNA [37]. With training, the exercise-induced mechanisms of muscle adaptation and repair may become attenuated. This is evidenced by reduced rates of muscle protein turnover in trained athletes [38]. Although, not assessed formally, the greatest increase in MGF after the exercise, was observed in those subjects who were least active [9]. The subjects who participated in the present study were recreationally active, undertaking some form of regular physical activity. Their low levels of MGF expression may reflect a relatively high level of fitness and consequently attenuation of adaptation responses to a single exercise bout. These observations highlight the importance of standardising training regimes and subject fitness levels for studies involving repair and adaptation pathways.
In conclusion, the present study shows that the regulation of IGF-I by rhGH differs in muscle and liver.
Although rhGH markedly increases IGF-I circulating levels, two weeks of rhGH administration did not upregulate skeletal muscle IGF-I mRNA in young males with normal GH endocrinology. Further studies would be required using larger subject numbers, higher doses or a longer dosing period, equivalent to that previously demonstrated to upregulate IGF-I expression in muscles of elderly men [17], to determine if GH has an effect on the IGF-I splice variants in young men.