You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Dat's - CJC-1295 & GHRP-6 (Basic Guides)
- Thread starter DatBtrue
- Start date
GoneForever
Banned
- Joined
- Jan 1, 1970
- Messages
- 4
Sweet, i'll up my dose from 3mg to 6mg now. I read some article where some doctors were saying that most melatonin has been extremely polluted. I guess there were major contanimants in it that have been causing a condition in people similar to polio or some shit like that. I'll try and find it. My dad actually found it so i'll see where he read it. But yah, it said to only use USP melatonin. It cannot come from china and many u.s. compaines will say its u.s. but really comes from China so you have to look for USP on the bottle. I went to bodybuilding.com and ordered Life Extensions Melatonin. Its very clean and dirt cheap.
GoneForever
Banned
- Joined
- Jan 1, 1970
- Messages
- 4
Man i was just reading an article that said glutamin raises insulin. Im shocked like %90 of everything we eat has such a damn effect on insulin. Makes it a bitch to try and time Gh the right way for fatloss.
- Joined
- Jul 25, 2008
- Messages
- 1,700
IGF-1 + IGFBP = localized action
Originally posted as post #635 on 10/26/08 in my AM thread.
Now I can get back to my theory that native IGF-1 either pre-complexed or coadministered with the binding protein IGFBP-3 will enhance local IGF-1 expression better then free IGF-1 and better then a long-lasting analog such as LR3.
Here are snippets from 2 studies I find interesting. The first indicates that IGF-1/IGFBP has a higher affinity to the area it is administered in ...but the reason does not mention size of the complex (which does also interest me) but rather IGFBP localizes IGF-1 to the cell "calling for it". This compares much more favorably then the tested use of LR3 in the study.
The second study mentions that IGF-1 coadministered w/ IGFBP OR IGF-1 coadministered with GH might give the optimal response.
Originally posted as post #644 on 10/29/08 in my AM thread.
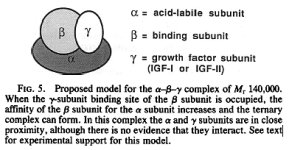
In the serum, the binary complex of IGF-IGFBP-3 can bind ALS (Acid-labile subunit), to form high molecular mass heterotrimeric complexes (195). These complexes are thought to prevent the efflux (a flowing outward) of the IGFs from the vasculature and hence play a crucial role in regulating IGF bioavailability to target tissues. - Cellular Actions of the Insulin-Like Growth Factor Binding Proteins, SUE M. FIRTH AND ROBERT C. BAXTER, Endocrine Reviews 2002 23(6):824–854
My Note: "In contrast to the IGFs and IGFBP-3, there is a substantial pool of free ALS in plasma which assures that IGF/IGFB-3 complex entering the circulation immediately forms the ternary complex." - United States Patent 5948757 http://www.freepatentsonline.com/5948757.html
Thus even though ALS contributes 63.3 kDa, to the 150-kDa ternary complex weight it is probably not necessary to prebind it to IGF-1 & IGFBP3 before injection.
It is probably sufficient to just inject prebound IGF-1 + IGBP3.
Originally posted as post #635 on 10/26/08 in my AM thread.
Now I can get back to my theory that native IGF-1 either pre-complexed or coadministered with the binding protein IGFBP-3 will enhance local IGF-1 expression better then free IGF-1 and better then a long-lasting analog such as LR3.
Here are snippets from 2 studies I find interesting. The first indicates that IGF-1/IGFBP has a higher affinity to the area it is administered in ...but the reason does not mention size of the complex (which does also interest me) but rather IGFBP localizes IGF-1 to the cell "calling for it". This compares much more favorably then the tested use of LR3 in the study.
The second study mentions that IGF-1 coadministered w/ IGFBP OR IGF-1 coadministered with GH might give the optimal response.
Clearance of IGFs and insulin from wounds: effect of IGF-binding protein interactions, J. Gray Robertson, Am J Physiol Endocrinol Metab 276:663-671, 1999.
...
Several earlier studies have attempted to measure the transfer of growth factors to tissues in vivo, with mixed success (18, 27). However, most of the data concerning the regulation of the movement of IGFs between extracellular compartments have been derived from plasma pharmacokinetic experiments that identify target organs or tissues but do not provide data on the extravascular kinetics of the IGFs. Analogs of IGF-I that exhibit poor binding characteristics to IGFBPs are more potent in vitro compared with native IGF-I (12), a potency that is attributed to reduced interactions with IGFBPs secreted into the media by cells in culture. However, these analogs are cleared from the circulation more quickly and are degraded to a greater extent than IGF-I (2), suggesting that IGFBPs may regulate the bioavailability of IGFs. The purpose of this study was to determine whether interactions with IGFBPs had any effect on the clearance of IGFs from wound sites. We have compared the rates of elimination and breakdown of IGF-I and IGF-II with an IGF analog that binds IGFBPs poorly, LR3-IGF-I (12), and insulin. We hypothesize that interactions with IGFBPs significantly decrease the rate of clearance of IGFs from wound sites.
...
From the Discussion:
...
Yet, positive responses to exogenous IGF-I have been equivocal and generally limited to those studies where an IGFBP was administered conjointly (16, 21, 42), pertubations of the growth hormone- IGF-IGFBP system exist (39, 42), or where the IGF is present at persistently increased levels (5, 7, 39). The roles of the IGFBPs that are present during wound repair and the parts they play in regulating IGF bioavailability are unclear. Nevertheless, some insight has been gained from observations of the effects of application of IGFBPs with IGF-I to wounds. Thus IGFBP-1 in the phosphorylated form has been shown to enhance wound epithelialization and granulation tissue formation when administered with IGF-I (21, 42), an effect that has been attributed to the ability of this IGFBP to associate with tissue integrins (13). Also, proteolytic fragments of IGFBP-3 have a reduced affinity for IGF-I and are thought to enhance IGF-I bioactivity by localizing IGF-I to the cell membrane (23, 32). This process has yet to be confirmed in vivo, although IGFBP-3 is proteolyzed in wound fluid (34), suggesting that such a mechanism does exist.
...
In conclusion, our observations that IGF analogs with reduced affinity for the IGFBPs are cleared from wound fluid at a greater rate than native IGF-I or IGF-II emphasize the importance of IGFBPs in maintaining a pool of IGF at the wound site.
Regulation of Insulin-Like Growth Factor I Bioavailability in Growing Animals, Jennifer M. Pell, J Anim Sci 1997. 75:20-31.
...Such encouraging findings have been applied to diet induced catabolism in humans and IGF-I administration improved nitrogen balance but tended to induce hypoglycemia and limit the therapeutic potential of IGF-I (Clemmons et al., 1992). These inappropriate actions of IGF-I probably occurred because the balance and structure of IGFBP, particularly IGFBP-3, are modified during many catabolic conditions so that exogenous IGF-I is more likely to remain in the free form, increasing vulnerability to undesirable side effects, inducing down-regulation of components of the IGF-I axis (such as IGFBP-3 synthesis), and being itself rapidly degraded. It has been suggested that an improved response might be obtained if IGF-I could be administered in a more physiological form, either precomplexed to an IGFBP or given in association with GH, which would maintain IGFBP-3 synthesis. Indeed, co-administration of IGF-I with either GH (Kupfer et al., 1993) or IGFBP-3 (Bagi et al., 1995) can, in certain circumstances, enhance IGF-I activity. These and other studies support the important function of the maintenance of IGF-I in a protein-bound form for optimum stability and activity (Lewitt et al., 1994). The data presented here using an antibody as a model IGF binding protein underscore the importance of circulating protein-bound IGF-I.
...
Several earlier studies have attempted to measure the transfer of growth factors to tissues in vivo, with mixed success (18, 27). However, most of the data concerning the regulation of the movement of IGFs between extracellular compartments have been derived from plasma pharmacokinetic experiments that identify target organs or tissues but do not provide data on the extravascular kinetics of the IGFs. Analogs of IGF-I that exhibit poor binding characteristics to IGFBPs are more potent in vitro compared with native IGF-I (12), a potency that is attributed to reduced interactions with IGFBPs secreted into the media by cells in culture. However, these analogs are cleared from the circulation more quickly and are degraded to a greater extent than IGF-I (2), suggesting that IGFBPs may regulate the bioavailability of IGFs. The purpose of this study was to determine whether interactions with IGFBPs had any effect on the clearance of IGFs from wound sites. We have compared the rates of elimination and breakdown of IGF-I and IGF-II with an IGF analog that binds IGFBPs poorly, LR3-IGF-I (12), and insulin. We hypothesize that interactions with IGFBPs significantly decrease the rate of clearance of IGFs from wound sites.
...
From the Discussion:
...
Yet, positive responses to exogenous IGF-I have been equivocal and generally limited to those studies where an IGFBP was administered conjointly (16, 21, 42), pertubations of the growth hormone- IGF-IGFBP system exist (39, 42), or where the IGF is present at persistently increased levels (5, 7, 39). The roles of the IGFBPs that are present during wound repair and the parts they play in regulating IGF bioavailability are unclear. Nevertheless, some insight has been gained from observations of the effects of application of IGFBPs with IGF-I to wounds. Thus IGFBP-1 in the phosphorylated form has been shown to enhance wound epithelialization and granulation tissue formation when administered with IGF-I (21, 42), an effect that has been attributed to the ability of this IGFBP to associate with tissue integrins (13). Also, proteolytic fragments of IGFBP-3 have a reduced affinity for IGF-I and are thought to enhance IGF-I bioactivity by localizing IGF-I to the cell membrane (23, 32). This process has yet to be confirmed in vivo, although IGFBP-3 is proteolyzed in wound fluid (34), suggesting that such a mechanism does exist.
...
In conclusion, our observations that IGF analogs with reduced affinity for the IGFBPs are cleared from wound fluid at a greater rate than native IGF-I or IGF-II emphasize the importance of IGFBPs in maintaining a pool of IGF at the wound site.
Regulation of Insulin-Like Growth Factor I Bioavailability in Growing Animals, Jennifer M. Pell, J Anim Sci 1997. 75:20-31.
...Such encouraging findings have been applied to diet induced catabolism in humans and IGF-I administration improved nitrogen balance but tended to induce hypoglycemia and limit the therapeutic potential of IGF-I (Clemmons et al., 1992). These inappropriate actions of IGF-I probably occurred because the balance and structure of IGFBP, particularly IGFBP-3, are modified during many catabolic conditions so that exogenous IGF-I is more likely to remain in the free form, increasing vulnerability to undesirable side effects, inducing down-regulation of components of the IGF-I axis (such as IGFBP-3 synthesis), and being itself rapidly degraded. It has been suggested that an improved response might be obtained if IGF-I could be administered in a more physiological form, either precomplexed to an IGFBP or given in association with GH, which would maintain IGFBP-3 synthesis. Indeed, co-administration of IGF-I with either GH (Kupfer et al., 1993) or IGFBP-3 (Bagi et al., 1995) can, in certain circumstances, enhance IGF-I activity. These and other studies support the important function of the maintenance of IGF-I in a protein-bound form for optimum stability and activity (Lewitt et al., 1994). The data presented here using an antibody as a model IGF binding protein underscore the importance of circulating protein-bound IGF-I.
Originally posted as post #644 on 10/29/08 in my AM thread.
In the serum, the binary complex of IGF-IGFBP-3 can bind ALS (Acid-labile subunit), to form high molecular mass heterotrimeric complexes (195). These complexes are thought to prevent the efflux (a flowing outward) of the IGFs from the vasculature and hence play a crucial role in regulating IGF bioavailability to target tissues. - Cellular Actions of the Insulin-Like Growth Factor Binding Proteins, SUE M. FIRTH AND ROBERT C. BAXTER, Endocrine Reviews 2002 23(6):824–854
195 - We have recently demonstrated that in the presence of IGF-I or IGF-II, the acid-stable and acid-labile proteins interact, resulting in the formation of one or more complexes of approximate 150,000-dalton , which dissociate irreversibly on acidification to yield free IGFs and one or more acid-stable IGF-binding protein (BP) species.
In conclusion, we have demonstrated that the growth hormone-dependent IGF-BP complex of Mr 140,000 in human serum can be reconstituted from three pure components, which we designate the a, b, and y subunits. Since the b subunit (BP-53) and y subunit (IGF-I or IGF-II) are present in the circulation in equimolar amounts (7), whereas the a subunit is present in excess (16), it would be expected that almost all circulating IGFs exist as part of this complex. How the IGFs are released from the complex to act on their target cells, and whether their transfer from the circulation to cells depends upon their binding to BP species derived from the complex, or to other BPs, remain important unanswered questions. - Structure of the Mr 140,000 growth hormone-dependent insulin-like growth factor binding protein complex: Determination by reconstitution and affinity-labeling, ROBERT C. BAXTER AND JANET L. MARTIN, Proc. Natl. Acad. Sci. USA Vol. 86, pp. 6898-6902, September 1989
In conclusion, we have demonstrated that the growth hormone-dependent IGF-BP complex of Mr 140,000 in human serum can be reconstituted from three pure components, which we designate the a, b, and y subunits. Since the b subunit (BP-53) and y subunit (IGF-I or IGF-II) are present in the circulation in equimolar amounts (7), whereas the a subunit is present in excess (16), it would be expected that almost all circulating IGFs exist as part of this complex. How the IGFs are released from the complex to act on their target cells, and whether their transfer from the circulation to cells depends upon their binding to BP species derived from the complex, or to other BPs, remain important unanswered questions. - Structure of the Mr 140,000 growth hormone-dependent insulin-like growth factor binding protein complex: Determination by reconstitution and affinity-labeling, ROBERT C. BAXTER AND JANET L. MARTIN, Proc. Natl. Acad. Sci. USA Vol. 86, pp. 6898-6902, September 1989
My Note: "In contrast to the IGFs and IGFBP-3, there is a substantial pool of free ALS in plasma which assures that IGF/IGFB-3 complex entering the circulation immediately forms the ternary complex." - United States Patent 5948757 http://www.freepatentsonline.com/5948757.html
Thus even though ALS contributes 63.3 kDa, to the 150-kDa ternary complex weight it is probably not necessary to prebind it to IGF-1 & IGFBP3 before injection.
It is probably sufficient to just inject prebound IGF-1 + IGBP3.
Last edited:
- Joined
- Jul 25, 2008
- Messages
- 1,700
Examples
Study #1
Study #1
Systemic administration of rhIGF-I or rhIGF-I/IGFBP-3 increases cortical bone and lean body mass in ovariectomized rats, Cedo M. Bagi, Bone Volume 16, Issue 4, Supplement 1, April 1995, Pages S263-S269
The purpose of this study was to compare dOselrelated effects on cortical bone and lean body mass following subcutaneous administration of rhIGF-I alone, or bound to an equimolar amount of rhIGFBP-3 to adult Ovx rats.
...
All rats treated with rhIGF-1 or the rhIGF-I/IGFBP13 complex had increased body weights, corresponding to a dose dependent increase in lean body mass.
...
A dramatic increase in periosteal, modeling- dependent formation, coupled with decreased or unchanged resorption on the endocortical envelope resulted in a dose-dependent increase in cortical thickness and crosssectional area in groups treated with the complex of rhIGF/IGFBP-3. This complex appeared to be more effective in promoting positive musculoskeletal changes than rhIGF-I alone. The potential of the rhIGF-I/IGFBP-3 complex to increase lean body mass and cortical bone thickness in Ovx rats deserves further evaluation as a candidate for treatment of musculoskeletal disorders in humans.
The purpose of this study was to compare dOselrelated effects on cortical bone and lean body mass following subcutaneous administration of rhIGF-I alone, or bound to an equimolar amount of rhIGFBP-3 to adult Ovx rats.
...
All rats treated with rhIGF-1 or the rhIGF-I/IGFBP13 complex had increased body weights, corresponding to a dose dependent increase in lean body mass.
...
A dramatic increase in periosteal, modeling- dependent formation, coupled with decreased or unchanged resorption on the endocortical envelope resulted in a dose-dependent increase in cortical thickness and crosssectional area in groups treated with the complex of rhIGF/IGFBP-3. This complex appeared to be more effective in promoting positive musculoskeletal changes than rhIGF-I alone. The potential of the rhIGF-I/IGFBP-3 complex to increase lean body mass and cortical bone thickness in Ovx rats deserves further evaluation as a candidate for treatment of musculoskeletal disorders in humans.
Last edited:
- Joined
- Jul 25, 2008
- Messages
- 1,700
Use of androgens increases local (as opposed to systemic) IGF-1 expression
Originally posted as post #634 on 10/26/08 in my AM thread.
An overview of the endocrinology of skeletal muscle , Melinda Sheffield-Moore and Randall J. Urban, TRENDS in Endocrinology and Metabolism Vol.15 No.3 April 2004
...
In young men made hypogonadal by administration of a gonadotropin-releasing hormone (GnRH) agonist, rhGH, increased concentrations of IGF1 mRNA in muscle biopsy samples obtained from the vastus lateralis [43]. In older men, administration of rhGH for one month also increased IGF1 mRNA concentrations in vastus lateralis muscle biopsy samples [44].
IGF1 expression is associated with skeletal muscle hypertrophy, as best demonstrated by animal studies where the IGF1 gene is selectively overexpressed in skeletal muscle [45]. Moreover, one mechanism by which IGF-I causes skeletal muscle hypertrophy might be through the stimulation of satellite cell replication; that is, by accelerating the progression of cell division [46].
There are many different triggers for local IGF1 expression, including androgens [47,13], mechanical load [48] and exercise [49]. Chronic inflammation, as indicated by interleukin-6 synthesis, is thought to cause loss of physical function by inhibiting local IGF1 skeletal muscle expression [50].
Originally posted as post #634 on 10/26/08 in my AM thread.
An overview of the endocrinology of skeletal muscle , Melinda Sheffield-Moore and Randall J. Urban, TRENDS in Endocrinology and Metabolism Vol.15 No.3 April 2004
...
In young men made hypogonadal by administration of a gonadotropin-releasing hormone (GnRH) agonist, rhGH, increased concentrations of IGF1 mRNA in muscle biopsy samples obtained from the vastus lateralis [43]. In older men, administration of rhGH for one month also increased IGF1 mRNA concentrations in vastus lateralis muscle biopsy samples [44].
IGF1 expression is associated with skeletal muscle hypertrophy, as best demonstrated by animal studies where the IGF1 gene is selectively overexpressed in skeletal muscle [45]. Moreover, one mechanism by which IGF-I causes skeletal muscle hypertrophy might be through the stimulation of satellite cell replication; that is, by accelerating the progression of cell division [46].
There are many different triggers for local IGF1 expression, including androgens [47,13], mechanical load [48] and exercise [49]. Chronic inflammation, as indicated by interleukin-6 synthesis, is thought to cause loss of physical function by inhibiting local IGF1 skeletal muscle expression [50].
46 - Fiorotto, M.L. et al. (2003) Persistent IGF-I overexpression in skeletal muscle transiently enhances DNA accretion and growth. FASEB J. 17, 59–60
47 - Sheffield-Moore, M. et al. (1999) Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J. Clin. Endocrinol. Metab. 84, 2705–2711
Androgens induce their specific response via the AR, which, in turn, regulates the transcription of androgen-responsive target genes. Although we know that accumulation of DNA is essential for muscle growth, the mechanisms of androgen-induced DNA accretion in skeletal muscle are unclear. AR (23) and AR mRNA (24) have been detected in human skeletal muscle. However, to date there are no human studies that have examined the response of skeletal muscle ARs to androgen exposure. Moreover, it has been suggested that prior cellular exposure to androgens may somehow prime these cells for the action of secondary agents such as IGF-I. Therefore, a secondary objective of this study was to examine the effect of oxandrolone (Anavar) administration on mRNA concentrations of IGF-I and ARs.
A recent study in exercising rats indicates that the accretion of skeletal muscle may be dependent on an increased number of ARs (25). Inoue et al. (25) examined the physiological importance of the increase in ARs on exercise-induced muscle hypertrophy. They determined that the androgen pathway had a significant effect on exercise-induced muscle hypertrophy and found the hypertrophy to be associated with an increased number of ARs in the exercised muscle (25). Moreover, a study conducted by Doumit et al. (26) found that pretreatment of porcine satellite cells with T for 24 h up-regulated AR, but did not alter the responsiveness of these cells to IGF-I or other growth factors. Similarly, we found an increased expression of AR mRNA with no change in im IGF-I mRNA concentrations after a short term administration of oxandrolone. These data along with our findings of increased mRNA concentrations of ARs with short term exposure to oxandrolone lend support to the contention that ARs may regulate, either directly or indirectly, the accumulation of DNA required for muscle growth.
More recent evidence lends support to the complementary roles of androgens, ARs, and IGF-I. Urban et al. (5) found increased mRNA concentrations of IGF-I in skeletal muscle of elderly men given 4 weeks of replacement doses of TE. Further, by inducing severe androgen deficiency in young men for 10 weeks, Mauras et al. (27) showed marked decreases in mRNA concentrations of IGF-I and suggested that within skeletal muscle tissue, androgens are necessary for local IGF-I production, independent of GH production and systemic IGF-I concentrations.
13 - Ferrando, A.A. et al. (2002) Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am. J. Physiol. Endocrinol. Metab. 282, E601–E607
Testosterone administration resulted in some noteworthy effects on AR and IGF-I expression in skeletal muscle. AR protein expression was increased after 1 mo of TE but had returned to pretreatment levels by 6 mo. Physiologically, it is logical that androgen would enhance its own receptor expression as it stimulates muscle metabolism. We previously noted an upregulation of AR expression with oxandrolone administration (18) in young males, which also occurred concomitantly with an increase in muscle protein synthesis. The return of AR expression to pretreatment values after 6 mo of continuous androgen administration indicates a steady-state adaptation to the treatment paradigm.
...
IGF-I accompanies increases in muscle mass and strength (17). In frail elderly, progressive resistance training that increases muscle mass and strength also increases intramuscular IGF-I concentrations (19). Clinically, we previously demonstrated that older men given testosterone for 1 mo increased IGF-I transcripts in muscle while decreasing the inhibitory IGF-binding protein (23). The present study agrees with our previous work in that IGF-I protein expression increased at 1 mo and further demonstrates that this increase was maintained throughout the 6 mo of testosterone administration. This confirms that the increase in IGF-I mRNA noted in our earlier study (23) translates into an actual increase of IGF-I protein. A corollary to these studies found that young men who were made hypogonadal for 10 wk by Lupron showed a decrease in muscle strength and a decrease in intramuscular IGF-I mRNA concentration (14). Taken together, these data indicate a mechanistic importance of IGF-I on muscle anabolism.
47 - Sheffield-Moore, M. et al. (1999) Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J. Clin. Endocrinol. Metab. 84, 2705–2711
Androgens induce their specific response via the AR, which, in turn, regulates the transcription of androgen-responsive target genes. Although we know that accumulation of DNA is essential for muscle growth, the mechanisms of androgen-induced DNA accretion in skeletal muscle are unclear. AR (23) and AR mRNA (24) have been detected in human skeletal muscle. However, to date there are no human studies that have examined the response of skeletal muscle ARs to androgen exposure. Moreover, it has been suggested that prior cellular exposure to androgens may somehow prime these cells for the action of secondary agents such as IGF-I. Therefore, a secondary objective of this study was to examine the effect of oxandrolone (Anavar) administration on mRNA concentrations of IGF-I and ARs.
A recent study in exercising rats indicates that the accretion of skeletal muscle may be dependent on an increased number of ARs (25). Inoue et al. (25) examined the physiological importance of the increase in ARs on exercise-induced muscle hypertrophy. They determined that the androgen pathway had a significant effect on exercise-induced muscle hypertrophy and found the hypertrophy to be associated with an increased number of ARs in the exercised muscle (25). Moreover, a study conducted by Doumit et al. (26) found that pretreatment of porcine satellite cells with T for 24 h up-regulated AR, but did not alter the responsiveness of these cells to IGF-I or other growth factors. Similarly, we found an increased expression of AR mRNA with no change in im IGF-I mRNA concentrations after a short term administration of oxandrolone. These data along with our findings of increased mRNA concentrations of ARs with short term exposure to oxandrolone lend support to the contention that ARs may regulate, either directly or indirectly, the accumulation of DNA required for muscle growth.
More recent evidence lends support to the complementary roles of androgens, ARs, and IGF-I. Urban et al. (5) found increased mRNA concentrations of IGF-I in skeletal muscle of elderly men given 4 weeks of replacement doses of TE. Further, by inducing severe androgen deficiency in young men for 10 weeks, Mauras et al. (27) showed marked decreases in mRNA concentrations of IGF-I and suggested that within skeletal muscle tissue, androgens are necessary for local IGF-I production, independent of GH production and systemic IGF-I concentrations.
13 - Ferrando, A.A. et al. (2002) Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am. J. Physiol. Endocrinol. Metab. 282, E601–E607
Testosterone administration resulted in some noteworthy effects on AR and IGF-I expression in skeletal muscle. AR protein expression was increased after 1 mo of TE but had returned to pretreatment levels by 6 mo. Physiologically, it is logical that androgen would enhance its own receptor expression as it stimulates muscle metabolism. We previously noted an upregulation of AR expression with oxandrolone administration (18) in young males, which also occurred concomitantly with an increase in muscle protein synthesis. The return of AR expression to pretreatment values after 6 mo of continuous androgen administration indicates a steady-state adaptation to the treatment paradigm.
...
IGF-I accompanies increases in muscle mass and strength (17). In frail elderly, progressive resistance training that increases muscle mass and strength also increases intramuscular IGF-I concentrations (19). Clinically, we previously demonstrated that older men given testosterone for 1 mo increased IGF-I transcripts in muscle while decreasing the inhibitory IGF-binding protein (23). The present study agrees with our previous work in that IGF-I protein expression increased at 1 mo and further demonstrates that this increase was maintained throughout the 6 mo of testosterone administration. This confirms that the increase in IGF-I mRNA noted in our earlier study (23) translates into an actual increase of IGF-I protein. A corollary to these studies found that young men who were made hypogonadal for 10 wk by Lupron showed a decrease in muscle strength and a decrease in intramuscular IGF-I mRNA concentration (14). Taken together, these data indicate a mechanistic importance of IGF-I on muscle anabolism.
- Joined
- Jul 25, 2008
- Messages
- 1,700
GH secretion rates
We have spent a few pages discussing making GH work for fat loss. It is interesting to take a look at GH and body growth to see how nature does it.
Its interesting that adults have more frequent pulses then growing adolescents with shorter interpulse intervals.
Growing adolescents have GH pulses of longer duration and higher amplitude. Each burst releases less or more than 10 times that of an adult depending on adolescent growing stage.
The daily production for GH in growing adolescents is very high in comparison to adults (exceeding a multiple of 20 at growth peak).
It is also interesting that GH production and burst intensity continues to escalate during the growing years before dropping substantially during the "coasting growth years" of post-puberty.
We have spent a few pages discussing making GH work for fat loss. It is interesting to take a look at GH and body growth to see how nature does it.
Its interesting that adults have more frequent pulses then growing adolescents with shorter interpulse intervals.
Growing adolescents have GH pulses of longer duration and higher amplitude. Each burst releases less or more than 10 times that of an adult depending on adolescent growing stage.
The daily production for GH in growing adolescents is very high in comparison to adults (exceeding a multiple of 20 at growth peak).
It is also interesting that GH production and burst intensity continues to escalate during the growing years before dropping substantially during the "coasting growth years" of post-puberty.
- Joined
- Jul 25, 2008
- Messages
- 1,700
Any clue on Post #442 (which was a response to #441)
Thanks man
Guanfacine as an alpha-2-agonist inducer of growth hormone secretion--a comparison with clonidine,J Balldin, U Berggren, E Eriksson, G Lindstedt, and A Sundkler, Psychoneuroendocrinology, January 1, 1993; 18(1): 45-55.
Doses of 0.5 mg and 1.0 mg of the alpha-2-adrenoceptor agonist guanfacine (GUA) and NaCl were administered intravenously (IV) in a randomized order to 18 healthy male subjects. GUA induced growth hormone (GH) secretion in a dose-dependent manner without affecting blood pressure or heart rate or inducing sedation. The effects of GUA 1.5 mg i.v. was compared with those of another alpha-2-adrenoceptor agonist, clonidine (CLON) 150 micrograms i.v. in six other male volunteers.
Both alpha-2-agonists increased GH to similar levels. CLON reduced both systolic and diastolic blood pressure levels, whereas GUA reduced only systolic levels. Sedation was significantly more pronounced after CLON. The results suggest that the GUA/GH-test (1.5 mg GUA i.v.) may be an alternative to the CLON/GH-test in neuroendocrine assessment of alpha-2-adrenoceptor sensitivity.
Doses of 0.5 mg and 1.0 mg of the alpha-2-adrenoceptor agonist guanfacine (GUA) and NaCl were administered intravenously (IV) in a randomized order to 18 healthy male subjects. GUA induced growth hormone (GH) secretion in a dose-dependent manner without affecting blood pressure or heart rate or inducing sedation. The effects of GUA 1.5 mg i.v. was compared with those of another alpha-2-adrenoceptor agonist, clonidine (CLON) 150 micrograms i.v. in six other male volunteers.
Both alpha-2-agonists increased GH to similar levels. CLON reduced both systolic and diastolic blood pressure levels, whereas GUA reduced only systolic levels. Sedation was significantly more pronounced after CLON. The results suggest that the GUA/GH-test (1.5 mg GUA i.v.) may be an alternative to the CLON/GH-test in neuroendocrine assessment of alpha-2-adrenoceptor sensitivity.
Usefulness of serotoninergic challenge with oral citalopram, P Mattos, VA Franco, F Noel, D Segenreich, and JC Goncalves Rev Bras Psiquiatr, September 1, 2006; 28(3): 203-5.
OBJECTIVE: Challenge tests designed to evaluate serotoninergic pathways have widely used intravenous citalopram. Oral citalopram has also been used, but unsatisfactory results were obtained with a dose of 20 mg. The objective of this study was to determine whether a higher oral dose would reproduce similar to those described for intravenous administration. To that end, we evaluated cortisol, growth hormone and prolactin levels.
METHOD: Eight healthy male volunteers were evaluated in a randomized crossover challenge test with 40 mg of oral citalopram or placebo.
RESULTS: Cortisol levels increased at 2-4h after the oral citalopram intake, with a small amplitude peak occurring in two-thirds of the subjects. Levels of prolactin and growth hormone remained unchanged throughout the study. CONCLUSION: The use of oral citalopram might present an alternative in serotoninergic challenge tests, but higher doses are required.
OBJECTIVE: Challenge tests designed to evaluate serotoninergic pathways have widely used intravenous citalopram. Oral citalopram has also been used, but unsatisfactory results were obtained with a dose of 20 mg. The objective of this study was to determine whether a higher oral dose would reproduce similar to those described for intravenous administration. To that end, we evaluated cortisol, growth hormone and prolactin levels.
METHOD: Eight healthy male volunteers were evaluated in a randomized crossover challenge test with 40 mg of oral citalopram or placebo.
RESULTS: Cortisol levels increased at 2-4h after the oral citalopram intake, with a small amplitude peak occurring in two-thirds of the subjects. Levels of prolactin and growth hormone remained unchanged throughout the study. CONCLUSION: The use of oral citalopram might present an alternative in serotoninergic challenge tests, but higher doses are required.
Guanfacine as an alpha-2-agonist inducer of growth hormone secretion--a comparison with clonidine,J Balldin, U Berggren, E Eriksson, G Lindstedt, and A Sundkler, Psychoneuroendocrinology, January 1, 1993; 18(1): 45-55.
Doses of 0.5 mg and 1.0 mg of the alpha-2-adrenoceptor agonist guanfacine (GUA) and NaCl were administered intravenously (IV) in a randomized order to 18 healthy male subjects. GUA induced growth hormone (GH) secretion in a dose-dependent manner without affecting blood pressure or heart rate or inducing sedation. The effects of GUA 1.5 mg i.v. was compared with those of another alpha-2-adrenoceptor agonist, clonidine (CLON) 150 micrograms i.v. in six other male volunteers.
Both alpha-2-agonists increased GH to similar levels. CLON reduced both systolic and diastolic blood pressure levels, whereas GUA reduced only systolic levels. Sedation was significantly more pronounced after CLON. The results suggest that the GUA/GH-test (1.5 mg GUA i.v.) may be an alternative to the CLON/GH-test in neuroendocrine assessment of alpha-2-adrenoceptor sensitivity.
Usefulness of serotoninergic challenge with oral citalopram, P Mattos, VA Franco, F Noel, D Segenreich, and JC Goncalves Rev Bras Psiquiatr, September 1, 2006; 28(3): 203-5.
OBJECTIVE: Challenge tests designed to evaluate serotoninergic pathways have widely used intravenous citalopram. Oral citalopram has also been used, but unsatisfactory results were obtained with a dose of 20 mg. The objective of this study was to determine whether a higher oral dose would reproduce similar to those described for intravenous administration. To that end, we evaluated cortisol, growth hormone and prolactin levels.
METHOD: Eight healthy male volunteers were evaluated in a randomized crossover challenge test with 40 mg of oral citalopram or placebo.
RESULTS: Cortisol levels increased at 2-4h after the oral citalopram intake, with a small amplitude peak occurring in two-thirds of the subjects. Levels of prolactin and growth hormone remained unchanged throughout the study. CONCLUSION: The use of oral citalopram might present an alternative in serotoninergic challenge tests, but higher doses are required.
Nice studies.
With citalopram, I've heard it can suppress REM sleep. Considering that babies spent a lot of time in REM sleep, it's possible that it could be important to growth (bone growth, muscle growth, recovery, etc...).
Also, anything on citalopram and IGF-1?
Last edited:
- Joined
- Jul 25, 2008
- Messages
- 1,700
Thanks. What about alcohol? How often and how much would it be safe to drink?
There's a bottle of cold Corona downstairs that I really want to have
Insulin-like growth factor (IGF)-1 and IGF-binding protein-1 concentrations in serum of normal subjects after alcohol ingestion: evidence for decreased IGF-1 bioavailability, Röjdmark, Rydvald, Aquilonius & Brismar, Clinical Endocrinology Volume 52 Issue 3, Pages 313 - 318
OBJECTIVE
Both insulin-like growth factor (IGF)-1 and insulin-like growth factor-binding protein (IGFBP)-1 are synthesized by the liver. It is well known that chronic alcohol abuse impairs liver function, but less is known about how an acute intake of moderate quantities of alcohol affects hepatic production of IGF-1 and IGFBP-1. The objective of the present investigation was to study this issue by measuring serum levels of IGF-1 and IGFBP-1 in normal subjects before, during and after ingestion of a moderate dose of ethanol.
SUBJECTS AND DESIGN
Eight healthy subjects were tested on two occasions. On one occasion (experiment A), three 150-ml doses of ordinary drinking-water were given at 08.00, 09.30, and 11.00 h. On another occasion (experiment B), three 150-ml drinks of diluted ethanol were given instead of water. Each drink contained 0.45 g ethanol/kg b.w. Experiments A and B were performed in random order, 1 week apart. Blood samples were collected before, during and after the drinks over a period of 7 h (08.00–15.00 h). One blood sample was also drawn at 08.00 h the following day.
MEASUREMENTS
Blood glucose and serum concentrations of ethanol, insulin, growth hormone (GH), IGF-1 and IGFBP-1 were determined.
RESULTS
When alcohol had been ingested, the serum ethanol concentration rose to a peak value of 28.6 ± 0.9 mmol/l (mean ± SEM). The serum IGF-1 level declined significantly toward the end of the 7-h period. The serum IGFBP-1 level increased promptly in response to ethanol, and reached a maximum 2.7 times above basal as the ethanol level peaked. Neither IGF-1, nor IGFBP-1 levels changed significantly after water intake. The IGF-1 : IGFBP-1 ratio declined markedly after ethanol (from 15.7 ± 3.9 to 3.9 ± 0.6; P < 0.01), but not after water intake. The blood glucose and serum levels of insulin and GH were unaffected by both ethanol and water.
CONCLUSIONS
Ingestion of moderate amounts of alcohol by healthy individuals results in an acute and profound increase in the serum IGFBP-1 level and a protracted and less powerful decline in the IGF-1 level. The mechanism behind the IGFBP-1 increase is probably a direct effect on the liver, since neither insulin, nor glucose or GH concentrations changed significantly in response to the ethanol challenge.
OBJECTIVE
Both insulin-like growth factor (IGF)-1 and insulin-like growth factor-binding protein (IGFBP)-1 are synthesized by the liver. It is well known that chronic alcohol abuse impairs liver function, but less is known about how an acute intake of moderate quantities of alcohol affects hepatic production of IGF-1 and IGFBP-1. The objective of the present investigation was to study this issue by measuring serum levels of IGF-1 and IGFBP-1 in normal subjects before, during and after ingestion of a moderate dose of ethanol.
SUBJECTS AND DESIGN
Eight healthy subjects were tested on two occasions. On one occasion (experiment A), three 150-ml doses of ordinary drinking-water were given at 08.00, 09.30, and 11.00 h. On another occasion (experiment B), three 150-ml drinks of diluted ethanol were given instead of water. Each drink contained 0.45 g ethanol/kg b.w. Experiments A and B were performed in random order, 1 week apart. Blood samples were collected before, during and after the drinks over a period of 7 h (08.00–15.00 h). One blood sample was also drawn at 08.00 h the following day.
MEASUREMENTS
Blood glucose and serum concentrations of ethanol, insulin, growth hormone (GH), IGF-1 and IGFBP-1 were determined.
RESULTS
When alcohol had been ingested, the serum ethanol concentration rose to a peak value of 28.6 ± 0.9 mmol/l (mean ± SEM). The serum IGF-1 level declined significantly toward the end of the 7-h period. The serum IGFBP-1 level increased promptly in response to ethanol, and reached a maximum 2.7 times above basal as the ethanol level peaked. Neither IGF-1, nor IGFBP-1 levels changed significantly after water intake. The IGF-1 : IGFBP-1 ratio declined markedly after ethanol (from 15.7 ± 3.9 to 3.9 ± 0.6; P < 0.01), but not after water intake. The blood glucose and serum levels of insulin and GH were unaffected by both ethanol and water.
CONCLUSIONS
Ingestion of moderate amounts of alcohol by healthy individuals results in an acute and profound increase in the serum IGFBP-1 level and a protracted and less powerful decline in the IGF-1 level. The mechanism behind the IGFBP-1 increase is probably a direct effect on the liver, since neither insulin, nor glucose or GH concentrations changed significantly in response to the ethanol challenge.
- Joined
- Jul 25, 2008
- Messages
- 1,700
Testosterone blunts IGF-1 inhibition of GH
Time to enlighten your sorry ass Dat.
Rexanator led me to Testosterone Blunts Feedback Inhibition of Growth Hormone Secretion by Experimentally Elevated Insulin-Like Growth Factor-I Concentration, Johannes D. Veldhuis, Stacey M. Anderson, Ali Iranmanesh and Cyril Y. Bowers, The Journal of Clinical Endocrinology & Metabolism Vol. 90, No. 3 1613-1617, 2005, where they found:
The results of this study were confirmed in a recent study published this month:
datBtrue said:I have no idea why some are posting that testosterone will stop IGF-1's multi-faceted inhibition of GH release. Perhaps someone can enlighten me?
Time to enlighten your sorry ass Dat.
Rexanator led me to Testosterone Blunts Feedback Inhibition of Growth Hormone Secretion by Experimentally Elevated Insulin-Like Growth Factor-I Concentration, Johannes D. Veldhuis, Stacey M. Anderson, Ali Iranmanesh and Cyril Y. Bowers, The Journal of Clinical Endocrinology & Metabolism Vol. 90, No. 3 1613-1617, 2005, where they found:
"...supplementation of a high dose of Te in middle-aged and older men attenuates IGF-I feedback-dependent inhibition of nadir and peak GH secretion."
The results of this study were confirmed in a recent study published this month:
Testosterone Supplementation in Older Men Restrains Insulin-Like Growth Factor’s Dose-Dependent Feedback Inhibition of Pulsatile Growth Hormone Secretion, Johannes D. Veldhuis, Daniel M. Keenan, Joy N. Bailey, Adenborduin Adeniji, John M. Miles, Remberto Paulo, Mihaela Cosma and Cacia Soares-Welch,The Journal of Clinical Endocrinology & Metabolism Vol. 94, No. 1 246-254, 2009
Background: Pulsatile GH secretion declines in older men. The causal mechanisms are unknown. Candidates include deficient feedforward (stimulation) by endogenous secretagogues and excessive feedback (inhibition) by GH or IGF-I due to age and/or relative hypoandrogenemia.
Hypothesis: Testosterone (T) supplementation in healthy older men will restrain negative feedback by systemic concentrations of IGF-I.
Subjects: Twenty-four healthy men (ages, 50 to 75 yr; body mass index, 24 to 30 kg/m2) participated in the study.
Methods: We performed a prospectively randomized, double-blind, placebo-controlled assessment of the impact of pharmacological T supplementation on GH responses to randomly ordered separate-day injections of recombinant human IGF-I doses of 0, 1.0, 1.5, and 2.0 mg/m2.
Analysis: Deconvolution and approximate entropy analyses of pulsatile, basal, and entropic (pattern-sensitive) modes of GH secretion were conducted.
Results: Recombinant human IGF-I injections 1) elevated mean and peak serum IGF-I concentrations dose-dependently (both P < 0.001); 2) suppressed pulsatile GH secretion (P = 0.003), burst mass (P = 0.025), burst number (P = 0.005), interpulse variability (P = 0.032), and basal GH secretion (P = 0.009); and 3) increased secretory pattern regularity (P = 0.020). T administration did not alter experimentally controlled IGF-I concentrations, but it elevated mean GH concentrations (P = 0.015) and stimulated pulsatile GH secretion (frequency P = 0.037, mass per burst P = 0.038). Compared with placebo, T attenuated exogenous IGF-I’s inhibition of GH secretory-burst mass (P < 0.038) without restoring pulse number, basal secretion, or pattern regularity.
Conclusion: The capability of systemic T to mute IGF-I feedback on pulsatile GH secretion suggests a novel mechanism for augmenting GH production.
Background: Pulsatile GH secretion declines in older men. The causal mechanisms are unknown. Candidates include deficient feedforward (stimulation) by endogenous secretagogues and excessive feedback (inhibition) by GH or IGF-I due to age and/or relative hypoandrogenemia.
Hypothesis: Testosterone (T) supplementation in healthy older men will restrain negative feedback by systemic concentrations of IGF-I.
Subjects: Twenty-four healthy men (ages, 50 to 75 yr; body mass index, 24 to 30 kg/m2) participated in the study.
Methods: We performed a prospectively randomized, double-blind, placebo-controlled assessment of the impact of pharmacological T supplementation on GH responses to randomly ordered separate-day injections of recombinant human IGF-I doses of 0, 1.0, 1.5, and 2.0 mg/m2.
Analysis: Deconvolution and approximate entropy analyses of pulsatile, basal, and entropic (pattern-sensitive) modes of GH secretion were conducted.
Results: Recombinant human IGF-I injections 1) elevated mean and peak serum IGF-I concentrations dose-dependently (both P < 0.001); 2) suppressed pulsatile GH secretion (P = 0.003), burst mass (P = 0.025), burst number (P = 0.005), interpulse variability (P = 0.032), and basal GH secretion (P = 0.009); and 3) increased secretory pattern regularity (P = 0.020). T administration did not alter experimentally controlled IGF-I concentrations, but it elevated mean GH concentrations (P = 0.015) and stimulated pulsatile GH secretion (frequency P = 0.037, mass per burst P = 0.038). Compared with placebo, T attenuated exogenous IGF-I’s inhibition of GH secretory-burst mass (P < 0.038) without restoring pulse number, basal secretion, or pattern regularity.
Conclusion: The capability of systemic T to mute IGF-I feedback on pulsatile GH secretion suggests a novel mechanism for augmenting GH production.
- Joined
- May 18, 2008
- Messages
- 323
I'm watchin' you bud 
Is this the same info you've got over at AM?
Is this the same info you've got over at AM?
- Joined
- Jul 25, 2008
- Messages
- 1,700
I'm watchin' you bud
Is this the same info you've got over at AM?
The early part of the thread probably is but within the last couple of months the two threads diverged.
The import things like the info on mod GRF(1-29) were carried over but plenty of things such as using GH to lose fat was not.
Some things get buried such as my post that injecting peg-MGF is probably just a "better" form of IGF-1 but that it has no way of entering the cell and doing what MGF does.
If people ever really understood the things I post about and all the studies I posted on AM they'd understand that GH, Insulin & Testosterone work really well together to increase MGF expression within the cell and muscle IGF-1 in the cell.
Instead they want to increase the amount of circulating IGF-1 which not only won't build muscle but in the long run reduces longevity.
Thats what caloric restriction, SIRT1, etc. effect... the pathways that IGF-1 promote...this blockage in turn promotes lifespan.
Last edited:
I've been looking into GHRP-6 and the fact that it has nice appetite stimulation properties. I've never ran anything other than injectable b-12 and wanted to know if there are any harsh side effects from it? I'm trying to put on weight and this seems like a great way to do it.
- Joined
- Jul 25, 2008
- Messages
- 1,700
I've been looking into GHRP-6 and the fact that it has nice appetite stimulation properties. I've never ran anything other than injectable b-12 and wanted to know if there are any harsh side effects from it? I'm trying to put on weight and this seems like a great way to do it.
Side-effects?
Not really. Above 100mcg cortisol and prolactin can become transiently elevated but still well within the normal range. This really isn't a problem though with any of the GHRPs except high doses of GHRP-2 and mod-high doses of Hexarelin.
If you want hunger then don't be afraid to double the dose of GHRP-6 to 200mcg.
GHRP-6 is the reference standard Growth Hormone Secretagogue. It was first created in the early 1980's by Bowers and has been used in many types of tests. It has even been used with success in human test subjects of 90+ years of age ...without significant negative side-effects.
Its probably one of the safest peptides you'll ever use.
You see it really is a synthetic derivative of the hormone Ghrelin without Ghrelin's lipogenic (ability to increase fat) effect. It was discover way before Ghrelin was identified and even before the Ghrelin-receptor was found.
That Ghrelin-receptor when discover (before Ghrelin) was named the Growth Hormone Sectretagogue Receptor (GHS-R) and is located predominantly on somatotroph cells in the Pituitary where GH is released.
But beyond the GH releasing effect in the Pituitary we find what appears to be many positive effects in other tissue as well. There are GHS-Rs in the Thymus and in other components of the immune system which at least in animal studies and in vitro results in youthful restoration & enhancement of the immune system.
There are GHS-Rs in various tissues that result in health benefit when a GHRP is used. For example:
Hexarelin in some circumstances is cardio protective. - Hexarelin protects rat cardiomyocytes from angiotensin II-induced apoptosis in vitro, Jin-Jiang Pang, Am J Physiol Heart Circ Physiol 286: H1063–H1069, 2004
GHRP-2 in some circumstances is liver protective. - GH-releasing peptide-2 administration prevents liver inflammatory response in endotoxemia, Miriam Granado, Am J Physiol Endocrinol Metab 294: E131–E141, 2008.
GHRP-6 is active in the brain protecting neurons from death. - Growth hormone releasing peptide-6 acts as a survival factor in glutamate-induced excitotoxicity, Arancha Delgado-Rubı´n de Celix, Journal of Neurochemistry, 2006, 99, 839–849
GHRP-2 in some circumstances is liver protective. - GH-releasing peptide-2 administration prevents liver inflammatory response in endotoxemia, Miriam Granado, Am J Physiol Endocrinol Metab 294: E131–E141, 2008.
GHRP-6 is active in the brain protecting neurons from death. - Growth hormone releasing peptide-6 acts as a survival factor in glutamate-induced excitotoxicity, Arancha Delgado-Rubı´n de Celix, Journal of Neurochemistry, 2006, 99, 839–849
GHRP-2 and it appears all the GHRPs are anti-catabolic. This property is unique to these peptides (is unrelated to the GH promoting effects) and in my opinion is AWESOME!
Ghrelin and I assume the GHRPs promote anabolism in a manner unrelated to GH via direct promote, differention & fusion of muscle cells. Again in my opinion this is AWESOME!
So to sum up mon bon homme. GHRP-6 est très beau. GHRP-6 n'est pas mal.
- Joined
- Jul 25, 2008
- Messages
- 1,700
GHRPs Anti-catabolic
As always I highlighted in red so if you want you can just read the red parts and get a gist of what the study found. However I encourage a full read as it is very easy to read and very cutting edge informative.
ANTI-CATABOLISM -> Ghrelin, GHRP-2, GHRP-6, Hexarelin <- ?
As always I highlighted in red so if you want you can just read the red parts and get a gist of what the study found. However I encourage a full read as it is very easy to read and very cutting edge informative.
ANTI-CATABOLISM -> Ghrelin, GHRP-2, GHRP-6, Hexarelin <- ?
GHRP-2, a GHS-R agonist, directly acts on myocytes to attenuate the dexamethasone-induced expressions of muscle-specific ubiquitin ligases, Atrogin-1 and MuRF1, Daisuke Yamamoto, et al., Life Sciences 82 (2008) 460–466
Introduction
A variety of diseases and conditions, including sepsis, cancer, renal failure, excess of glucocorticoid, denervation and disuse of muscle, can cause muscle atrophy. In these diverse conditions, the atrophying muscles show increased protein degradation through activation of the ubiquitin (Ub)-proteasome pathway (Baracos et al., 1995; Kayali et al., 1987; Price et al., 1996; Tiao et al., 1997; Tischler et al., 1990). It is recently reported that the expressions of Atrogin-1 and MuRF1, both of which are musclespecific Ub-ligases, are involved in protein degradation in muscle and increased in these diverse conditions causing muscle atrophy (Bodine et al., 2001; Gomes et al., 2001; Lecker et al., 2004). Atrogin-1 is a muscle-specific F-box type E3 ligase and reported to be induced 8 to 40 fold in muscle atrophy during fasting, diabetes, cancer and renal failure (Bodine et al., 2001), up to 3 fold in hind limb suspension, immobilization and denervation, and up to 10 fold in cachetic or dexamethasone administration model (Gomes et al., 2001). MuRF1 is a Ring Finger type muscle-specific E3 ligase that is initially found in association with the myofibril (Kandarian and Jackman, 2006) and suggested to play an important role in the myofibrillar proteins breakdown. Both muscle-specific E3 ligases are considered to play a pivotal role in muscle atrophy because knockout mice lacking these E3 ligases are prevented from muscle atrophy (56% sparing for atrogin-1-/- and 36% for MuRF1-/-) (Bodine et al., 2001).
On the other hand, several protective factors for muscle atrophy have been reported. One of the potent protective factors is IGF-I. IGF-I prevents muscle atrophy induced by glucocorticoid (Kanda et al., 1999; Schakman et al., 2005), disuse (Alzghoul et al., 2004) and denervation (Day et al., 2002). IGF-I has a potency to inhibit Atrogin-1 and MuRF1 expressions in atrophying muscle (Sacheck et al., 2004; Stitt et al., 2004). The protective effect of IGF-I for muscle atrophy, at least partly, is exerted by this mechanism (Bodine et al., 2001; Lecker et al., 2004).
Ghrelin stimulates GH release from the pituitary through the GH secretagogue receptor (GHS-R) (Kojima et al., 1999). Also, Growth Hormone Releasing Peptide-2 (GHRP-2), a synthetic ligand for GHS-R, stimulates GH release from the pituitary (Wu et al., 1996). GHRP-2 administration increases plasma GH levels in rats (Sawada et al., 1994) and humans (Pihoker et al., 1995). As a result, plasma IGF-I levels are reported to increase in some studies (Bowers et al., 2004). Thus, GHRP-2 is expected to have a protective action against muscle atrophy via IGF-I. Indeed, a recent report suggested that GHRP-2 was able to prevent arthritis-induced increase in Atrogin-1 and MuRF1 expressions in rat muscle (Granado et al., 2005).
On the other hand, there are reports suggesting the presence of GHS-R in muscle (Papotti et al., 2000; Pierno et al., 2003) and the signal transduction mechanism of ghrelin is partly similar to those of IGF-I and insulin (Murata et al., 2002). Hence GHS-R ligands may play a role in the process of muscle atrophy.
In the present study, we have examined the effect of GHRP-2 on Atrogin-1 and MuRF1 mRNA levels in dexamethasoneinduced muscle atrophy in the rats, as a model of muscle atrophy that is often observed during steroid hormone-treatment in human. We have further tested whether the effect is a direct action on myocytes through GHS-R and found for the first time that GHRP-2 directly acted on myocytes.
...
Discussion
In the present experiment, we found that GHRP-2 attenuated Atrogin-1 mRNA level induced by dexamethasone in ratmuscles. Although the mechanism by which dexamethasone causes muscle atrophy is unknown, one possibility is via enhancement of glutamine synthetase activity (Falduto et al., 1992a,b) and the other is via induction of Atrogin-1 expression (Bodine et al., 2001; Lecker et al., 2004).
GHRP-2 has an action to stimulate GH secretion from pituitary, which in turn could increase plasma IGF-I levels. Since IGF-I has been reported to be a growth factor causing muscle hypertrophy (Kanda et al., 1999; Schakman et al., 2005), the elevation of plasma IGF-I levels may affect dexamethasoneinduced muscle atrophy. Interestingly IGF-I has been already reported to attenuate Atrogin-1 expression in vivo (Sacheck et al., 2004; Stitt et al., 2004). In the present study, however, plasma IGF-I levels were not changed by the treatment with GHRP-2. This finding was consistent with previous reports that GHRP-2 did not increase plasma IGF-I levels in mice (Tschop et al., 2002) and humans (Nijland et al., 1998), suggesting that GHRP-2 does not always increase plasma IGF-I levels. Our data rather suggested that the reduced mRNA levels of Atrogin-1 and MuRF1 in muscle by GHRP-2 was not due to the rise of circulating IGF-I levels. In addition, IGF-I expression in soleus muscles was not affected by GHRP-2 in the present study. Recently, Granado et al. (2005) reported that subcutaneous daily administration of GHRP-2 (100 ug/kg) decreased expression of Atrogin-1 and MuRF1 in atrophic muscle of adjuvant-induced arthritis rats. In their report, plasma IGF-I level was much lower in arthritis rats than in normal control and GHRP-2 did not increase muscle IGF-I mRNA level. Their findings, consistent with our findings, suggested that GHRP-2 decreased Atrogin-1 and MuRF1 mRNA levels through a pathway other than circulating IGF-I and local IGF-I production.
Binding assay using GHS-R ligands has shown specific binding sites in muscle (Papotti et al., 2000) and in vitro application of ghrelin or ghrelin agonists modulated chloride and potassium conductance in rat muscle (Pierno et al., 2003). These findings suggest the presence of GHS-R in skeletal muscle. In this experiment, we found the expression of GHSR-1a in differentiated C2C12 cells. We have already reported that intracellular signal pathways of ghrelin were partly similar to those of insulin and IGF-I (Murata et al., 2002). From the above reasons, we speculated GHRP-2 might work in myocytes to suppress Atrogin-1 and MuRF1 mRNA levels like IGF-I and examined whether GHRP-2 has a direct action on myocytes to inhibit Atrogin-1 and MuRF1 mRNA expressions. GHRP-2 dose-dependently suppressed dexamethasone-induced Atrogin- 1 and MuRF1 expressions in C2C12 cells. These findings indicate that GHRP-2 directly acts on myocytes and attenuates the level of Atrogin-1 and MuRF1 mRNA.
To further clarify a direct suppressive effect of GHRP-2 on Atrogin-1 and MuRF1 mRNA levels, [D-Lys3]-GHRP-6, a GHS-R-1a antagonist was used in C2C12 cells. There are two types of ghrelin receptors, GHS-R-1a and GHS-R-1b (Howard et al., 1996; Mckee et al., 1997). GHS-R-1a is an active receptor mediating ghrelin action. GHS-R-1b, a splicing variant of GHSR-1a, does not mediate ghrelin signal. We examined the specificity of GHRP-2 action using [D-Lys3]-GHRP-6. We found that [D-Lys3]-GHRP-6 partly and completely reversed the suppressive effects of GHRP-2 on Atrogin-1 and MuRF1 mRNA levels, respectively. These results suggest that GHRP-2 directly inhibits Atrogin-1 and MuRF1 mRNA level through GHS-R-1a.
Since C2C12 cells produce IGF-I (Frost et al., 2003), paracrine or autocrine action of IGF-I may be involved in the suppressive effect of GHRP-2 on Atrogin-1 and MuRF1 mRNA level. To elucidate this possibility, we measured IGF-I mRNA level in C2C12 cells. However, we were not able to find the increase in IGF-I mRNA in C2C12 cells in response to GHRP-2, suggesting that locally produced-IGF-I in C2C12 cells is not involved in the suppressive effect of GHRP-2 on Atrogin-1 and MuRF1 mRNA levels. Dexamethasone also did not influence IGF-I mRNA level in C2C12 cells, although it decreased IGF-I mRNA level in vivo soleus muscle. These results suggest that dexamethasone has an indirect action to reduce IGF-I mRNA level in muscles in in vivo animals. Glucocorticoid is reported to inhibit pulsatile GH secretion (Giustina and Veldhuis, 1998) and reduce GH receptor expression (King and Carter-Su, 1995). As a result, IGF-I mRNA level was thought to decrease in vivo experiment in the present study. Dexamethasone has been reported to reduce the expression in in vivo animals (Gilson et al., 2007), being consistent with our in vivo result.
In summary, GHRP-2 suppressed dexamethasone-induced Atrogin-1 mRNA expressions in in vivo rats without elevating plasma IGF-I and IGF-I mRNA in muscle. Furthermore GHRP-2 decreased dexamethasone-induced Atrogin-1 and MuRF1 expressions in C2C12 myocytes. This effect was blocked by the addition of [D-Lys3]-GHRP-6, a GHS-R-1a antagonist. These findings suggest that a direct action of GHRP-2 through GHSR-1a suppresses Atrogin-1 and MuRF1 mRNA levels in C2C12 cells. GHRP-2 might lead to the protection of muscle atrophy induced by dexamethasone.
REFERENCES
Alzghoul, M.B., Gerrard, D., Watkins, B.A., Hannon, K., 2004. Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. The FASEB Journal 18 (1), 221–223.
Baracos, V.E., Devivo, C., Hoyle, D.H., Goldberg, A.L., 1995. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. The American Journal of Physiology-Endocrinology and Metabolism 268 (5 Pt 1), E996–E1006.
Bodine, S.C., Latres, E., Baumhueter, S., Lai, V.K., Nunez, L., Clarke, B.A., Poueymirou,W.T., Panaro, F.J.,Na,E.,Dharmarajan,K., Pan, Z.Q.,Valenzuela, D.M., Dechiara, T.M., Stitt, T.N., Yancopoulos, G.D., Glass, D.J., 2001. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294 (5547), 1704–1708.
Bowers, C.Y., Granda, R., Mohan, S., Kuipers, J., Baylink, D., Veldhuis, J.D., 2004. Sustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and women. The Journal of Clinical Endocrinology and Metabolism 89 (5), 2290.2300.
Cheng, K., Chan, W.W., Barreto Jr., A., Convey, E.M., Smith, R.G., 1989. The synergistic effects of His-D-Trp-Ala-Trp-D-Phe-Lys-NH2 on growth hormone (GH)-releasing factor-stimulated GH release and intracellular adenosine 3' 5' -monophosphate accumulation in rat primary pituitary cell culture. Endocrinology 124, 2791.2798.
Day, C.S., Buranapanitkit, B., Riano, F.A., Tomaino, M.M., Somogyi, G., Sotereanos, D.G., Kuroda, R., Huard, J., 2002. Insulin growth factor-1 decreases muscle atrophy following denervation. Microsurgery 22 (4),
144.151. Falduto, M.T., Young, A.P., Hickson, R.C., 1992a. Exercise inhibits glucocorticoid- induced glutamine synthetase expression in red skeletal muscles. American Journal of Physiology-Cell Physiology 262 (1 Pt 1), C214.C220.
Falduto, M.T., Young, A.P., Hickson, R.C., 1992b. Exercise interrupts ongoing glucocorticoid-induced muscle atrophy and glutamine synthetase induction. American Journal of Physiology-Cell Physiology 263 (6 Pt 1), E1157.E1163.
Frost, R.A., Nystrom, G.J., Lang, C.H., 2003. Tumor necrosis factor-alpha decreases insulin-like growth factor-I messenger ribonucleic acid expression in C2C12 myoblasts via a Jun N-terminal kinase pathway. Endocrinology 144 (5), 1770.1779.
Gilson, H., Schakman, O., Combaret, L., Lause, P., Grobet, L., Attaix, D., Ketelslegers, J.M., Thissen, J.P., 2007. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 148 (1), 452.460.
Giustina, A., Veldhuis, J.D., 1998. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocrine Reviews 19 (6), 717.797.
Gomes, M.D., Lecker, S.H., Jagoe, R.T., Navon, A., Goldberg, A.L., 2001. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proceedings of the National Academy of Sciences of the United States of America 98 (25), 14440.14445.
Granado, M., Priego, T., Martin, A.I., Villanua, M.A., Lopez-Calderon, A., 2005. Ghrelin receptor agonist GHRP-2 prevents arthritis-induced increase in E3 ubiquitin-ligating enzymes MuRF1 and MAFbx gene expression in skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism 289 (6), E1007.E1014.
Howard, A.D., Feighner, S.D., Cully, D.F., Arena, J.P., Liberator, P.A., Rosenblum, C.I., Hamelin, M., Hreniuk, D.L., Palyha, O.C., Anderson, J., Paress, P.S., Diaz, C., Chou, M., Liu, K.K., Mckee, K.K., Pong, S.S., Chaung, L.Y., Elbrecht, A., Dashkevicz, M., Heavens, R., Rigby, M., Sirinathsinghji, D.J., Dean, D.C., Melillo, D.G., Patchett, A.A., Nargund, R., Griffin, P.R., Demartino, J.A., Gupta, S.K., Schaeffer, J.M., Smith, R.G., Van Der Ploeg, L.H., 1996. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273 (5277), 974.977.
Kanda, F., Takatani, K., Okuda, S., Matsushita, T., Chihara, K., 1999. Preventive effects of insulinlike growth factor-I on steroid-induced muscle atrophy. Muscle & Nerve 22 (2), 213.217.
Kandarian, S.C., Jackman, R.W., 2006. Intracellular signaling during skeletal muscle atrophy. Muscle & Nerve 33 (2), 155.165.
Kayali, A.G., Young, V.R., Goodman, M.N., 1987. Sensitivity of myofibrillar proteins to glucocorticoid-induced muscle proteolysis. American Journal of Physiology-Endocrinology and Metabolism 252 (5 Pt 1), E621.E626.
King, A.P., Carter-Su, C., 1995. Dexamethasone-induced antagonism of growth hormone (GH) action by down-regulation of GH binding in 3T3-F442A fibroblasts. Endocrinology 136 (11), 4796.4803.
Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H., Kangawa, K., 1999. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402 (6762), 656.660.
Lecker, S.H., Jagoe, R.T., Gilbert, A., Gomes, M., Baracos, V., Bailey, J., Price, S.R., Mitch, W.E., Goldberg, A.L., 2004. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. The FASEB journal 18 (1), 39.51.
Ma, K., Mallidis, C., Bhasin, S., Mahabadi, V., Artaza, J., Gonzalez-Cadavid, N., Arias, J., Salehian, B., 2003. Glucocorticoid-induced skeletal muscleatrophy is associated with upregulation of myostatin gene expression. American Journal of Physiology-Endocrinology and Metabolism 285 (2), E363–E371.
Mckee, K.K., Palyha, O.C., Feighner, S.D., Hreniuk, D.L., Tan, C.P., Phillips, M.S., Smith, R.G., Van Der Ploeg, L.H., Howard, A.D., 1997. Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Molecular Endocrinology 11 (4), 415–423.
Murata, M., Okimura, Y., Iida, K., Matsumoto, M., Sowa, H., Kaji, H., Kojima, M., Kangawa, K., Chihara, K., 2002. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. The Journal of Biological Chemistry 277 (7), 5667–5674.
Nijland, E.A., Strasburger, C.J., Popp-Snijders, C., Van Der Wal, P.S., Van Der Veen, E.A., 1998. A five day treatment with daily subcutaneous injections of growth hormone-releasing peptide-2 causes response attenuation and does not stimulate insulin-like growth factor-I secretion in healthy young men. European Journal of Endocrinology 139 (4), 395–401.
Papotti, M., Ghe, C., Cassoni, P., Catapano, F., Deghenghi, R., Ghigo, E., Muccioli, G., 2000. Growth hormone secretagogue binding sites in peripheral human tissues. The Journal of Clinical Endocrinology and Metabolism 85 (10), 3803–3807.
Pierno, S., De Luca, A., Desaphy, J.F., Fraysse, B., Liantonio, A., Didonna, M.P., Lograno, M., Cocchi, D., Smith, R.G., Camerino, D.C., 2003. Growth hormone secretagogues modulate the electrical and contractile properties of rat skeletal muscle through a ghrelin-specific receptor. British Journal of Pharmacology 139 (3), 575–584.
Pihoker, C., Middleton, R., Reynolds, G.A., Bowers, C.Y., Badger, T.M., 1995. Diagnostic studies with intravenous and intranasal growth hormonereleasing peptide-2 in children of short stature. The Journal of Clinical Endocrinology Metabolism 80 (10), 2987–2992.
Price, S.R., Bailey, J.L., England, B.K., 1996. Necessary but not sufficient: the role of glucocorticoids in the acidosis-induced increase in levels of mRNAs encoding proteins of the ATP-dependent proteolytic pathway in rat muscle. Mineral Electrolyte Metabolism 22 (1–3), 72–75.
Sacheck, J.M., Ohtsuka, A., Mclary, S.C., Goldberg, A.L., 2004. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. American Journal of Physiology-Endocrinology and Metabolism 287 (4), E591.E601.
Sawada, H., Sugihara, H., Onose, H., Minami, S., Shibasaki, T., Wakabayashi, I., 1994. Effect of D-Ala-D-beta Nal-Ala-Trp-D-Phe-Lys-NH2 (KP-102) on GH secretion in urethan-anesthetized rats. Regulatory Peptides 53 (3), 195.201.
Schakman, O., Gilson, H., De Coninck, V., Lause, P., Verniers, J., Havaux, X., Ketelslegers, J.M., Thissen, J.P., 2005. Insulin-like growth factor-I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid- treated rats. Endocrinology 146 (4), 1789.1797.
Smith, R.G., Cheng, K., Schoen,W.R., Pong, S.S., Hickey, G., Jacks, T., Butler, B., Chan, W.W., Chaung, L.Y., Judith, F., 1993. A nonpeptidyl growth hormone secretagogue. Science 260 (5114), 1640.1643.
Stitt, T.N., Drujan, D., Clarke, B.A., Panaro, F., Timofeyva, Y., Kline, W.O., Gonzalez, M., Yancopoulos, G.D., Glass, D.J., 2004. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Molecular Cell 14 (3), 395.403.
Tiao, G., Hobler, S., Wang, J.J., Meyer, T.A., Luchette, F.A., Fischer, J.E., Hasselgren, P.O., 1997. Sepsis is associated with increased mRNAs of the ubiquitin-proteasome proteolytic pathway in human skeletal muscle. Journal of Clinical Investigation 99 (2), 163.168.
Tischler, M.E., Rosenberg, S., Satarug, S., Henriksen, E.J., Kirby, C.R., Tome, M., Chase, P., 1990. Different mechanisms of increased proteolysis in atrophy induced by denervation or unweighting of rat soleus muscle. Metabolism 39 (7), 756.763.
Tschop, M., Statnick, M.A., Suter, T.M., Heiman, M.L., 2002. GH-releasing peptide-2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology 143 (2), 558.568.
Wu, D., Chen, C., Zhang, J., Bowers, C.Y., Clarke, I.J., 1996. The effects of GH-releasing peptide-6 (GHRP-6) and GHRP-2 on intracellular adenosine 3' 5' -monophosphate (cAMP) levels and GH secretion in ovine and rat somatotrophs. Journal of Endocrinology 148 (2), 197.205.
Introduction
A variety of diseases and conditions, including sepsis, cancer, renal failure, excess of glucocorticoid, denervation and disuse of muscle, can cause muscle atrophy. In these diverse conditions, the atrophying muscles show increased protein degradation through activation of the ubiquitin (Ub)-proteasome pathway (Baracos et al., 1995; Kayali et al., 1987; Price et al., 1996; Tiao et al., 1997; Tischler et al., 1990). It is recently reported that the expressions of Atrogin-1 and MuRF1, both of which are musclespecific Ub-ligases, are involved in protein degradation in muscle and increased in these diverse conditions causing muscle atrophy (Bodine et al., 2001; Gomes et al., 2001; Lecker et al., 2004). Atrogin-1 is a muscle-specific F-box type E3 ligase and reported to be induced 8 to 40 fold in muscle atrophy during fasting, diabetes, cancer and renal failure (Bodine et al., 2001), up to 3 fold in hind limb suspension, immobilization and denervation, and up to 10 fold in cachetic or dexamethasone administration model (Gomes et al., 2001). MuRF1 is a Ring Finger type muscle-specific E3 ligase that is initially found in association with the myofibril (Kandarian and Jackman, 2006) and suggested to play an important role in the myofibrillar proteins breakdown. Both muscle-specific E3 ligases are considered to play a pivotal role in muscle atrophy because knockout mice lacking these E3 ligases are prevented from muscle atrophy (56% sparing for atrogin-1-/- and 36% for MuRF1-/-) (Bodine et al., 2001).
On the other hand, several protective factors for muscle atrophy have been reported. One of the potent protective factors is IGF-I. IGF-I prevents muscle atrophy induced by glucocorticoid (Kanda et al., 1999; Schakman et al., 2005), disuse (Alzghoul et al., 2004) and denervation (Day et al., 2002). IGF-I has a potency to inhibit Atrogin-1 and MuRF1 expressions in atrophying muscle (Sacheck et al., 2004; Stitt et al., 2004). The protective effect of IGF-I for muscle atrophy, at least partly, is exerted by this mechanism (Bodine et al., 2001; Lecker et al., 2004).
Ghrelin stimulates GH release from the pituitary through the GH secretagogue receptor (GHS-R) (Kojima et al., 1999). Also, Growth Hormone Releasing Peptide-2 (GHRP-2), a synthetic ligand for GHS-R, stimulates GH release from the pituitary (Wu et al., 1996). GHRP-2 administration increases plasma GH levels in rats (Sawada et al., 1994) and humans (Pihoker et al., 1995). As a result, plasma IGF-I levels are reported to increase in some studies (Bowers et al., 2004). Thus, GHRP-2 is expected to have a protective action against muscle atrophy via IGF-I. Indeed, a recent report suggested that GHRP-2 was able to prevent arthritis-induced increase in Atrogin-1 and MuRF1 expressions in rat muscle (Granado et al., 2005).
On the other hand, there are reports suggesting the presence of GHS-R in muscle (Papotti et al., 2000; Pierno et al., 2003) and the signal transduction mechanism of ghrelin is partly similar to those of IGF-I and insulin (Murata et al., 2002). Hence GHS-R ligands may play a role in the process of muscle atrophy.
In the present study, we have examined the effect of GHRP-2 on Atrogin-1 and MuRF1 mRNA levels in dexamethasoneinduced muscle atrophy in the rats, as a model of muscle atrophy that is often observed during steroid hormone-treatment in human. We have further tested whether the effect is a direct action on myocytes through GHS-R and found for the first time that GHRP-2 directly acted on myocytes.
...
Discussion
In the present experiment, we found that GHRP-2 attenuated Atrogin-1 mRNA level induced by dexamethasone in ratmuscles. Although the mechanism by which dexamethasone causes muscle atrophy is unknown, one possibility is via enhancement of glutamine synthetase activity (Falduto et al., 1992a,b) and the other is via induction of Atrogin-1 expression (Bodine et al., 2001; Lecker et al., 2004).
GHRP-2 has an action to stimulate GH secretion from pituitary, which in turn could increase plasma IGF-I levels. Since IGF-I has been reported to be a growth factor causing muscle hypertrophy (Kanda et al., 1999; Schakman et al., 2005), the elevation of plasma IGF-I levels may affect dexamethasoneinduced muscle atrophy. Interestingly IGF-I has been already reported to attenuate Atrogin-1 expression in vivo (Sacheck et al., 2004; Stitt et al., 2004). In the present study, however, plasma IGF-I levels were not changed by the treatment with GHRP-2. This finding was consistent with previous reports that GHRP-2 did not increase plasma IGF-I levels in mice (Tschop et al., 2002) and humans (Nijland et al., 1998), suggesting that GHRP-2 does not always increase plasma IGF-I levels. Our data rather suggested that the reduced mRNA levels of Atrogin-1 and MuRF1 in muscle by GHRP-2 was not due to the rise of circulating IGF-I levels. In addition, IGF-I expression in soleus muscles was not affected by GHRP-2 in the present study. Recently, Granado et al. (2005) reported that subcutaneous daily administration of GHRP-2 (100 ug/kg) decreased expression of Atrogin-1 and MuRF1 in atrophic muscle of adjuvant-induced arthritis rats. In their report, plasma IGF-I level was much lower in arthritis rats than in normal control and GHRP-2 did not increase muscle IGF-I mRNA level. Their findings, consistent with our findings, suggested that GHRP-2 decreased Atrogin-1 and MuRF1 mRNA levels through a pathway other than circulating IGF-I and local IGF-I production.
Binding assay using GHS-R ligands has shown specific binding sites in muscle (Papotti et al., 2000) and in vitro application of ghrelin or ghrelin agonists modulated chloride and potassium conductance in rat muscle (Pierno et al., 2003). These findings suggest the presence of GHS-R in skeletal muscle. In this experiment, we found the expression of GHSR-1a in differentiated C2C12 cells. We have already reported that intracellular signal pathways of ghrelin were partly similar to those of insulin and IGF-I (Murata et al., 2002). From the above reasons, we speculated GHRP-2 might work in myocytes to suppress Atrogin-1 and MuRF1 mRNA levels like IGF-I and examined whether GHRP-2 has a direct action on myocytes to inhibit Atrogin-1 and MuRF1 mRNA expressions. GHRP-2 dose-dependently suppressed dexamethasone-induced Atrogin- 1 and MuRF1 expressions in C2C12 cells. These findings indicate that GHRP-2 directly acts on myocytes and attenuates the level of Atrogin-1 and MuRF1 mRNA.
To further clarify a direct suppressive effect of GHRP-2 on Atrogin-1 and MuRF1 mRNA levels, [D-Lys3]-GHRP-6, a GHS-R-1a antagonist was used in C2C12 cells. There are two types of ghrelin receptors, GHS-R-1a and GHS-R-1b (Howard et al., 1996; Mckee et al., 1997). GHS-R-1a is an active receptor mediating ghrelin action. GHS-R-1b, a splicing variant of GHSR-1a, does not mediate ghrelin signal. We examined the specificity of GHRP-2 action using [D-Lys3]-GHRP-6. We found that [D-Lys3]-GHRP-6 partly and completely reversed the suppressive effects of GHRP-2 on Atrogin-1 and MuRF1 mRNA levels, respectively. These results suggest that GHRP-2 directly inhibits Atrogin-1 and MuRF1 mRNA level through GHS-R-1a.
Since C2C12 cells produce IGF-I (Frost et al., 2003), paracrine or autocrine action of IGF-I may be involved in the suppressive effect of GHRP-2 on Atrogin-1 and MuRF1 mRNA level. To elucidate this possibility, we measured IGF-I mRNA level in C2C12 cells. However, we were not able to find the increase in IGF-I mRNA in C2C12 cells in response to GHRP-2, suggesting that locally produced-IGF-I in C2C12 cells is not involved in the suppressive effect of GHRP-2 on Atrogin-1 and MuRF1 mRNA levels. Dexamethasone also did not influence IGF-I mRNA level in C2C12 cells, although it decreased IGF-I mRNA level in vivo soleus muscle. These results suggest that dexamethasone has an indirect action to reduce IGF-I mRNA level in muscles in in vivo animals. Glucocorticoid is reported to inhibit pulsatile GH secretion (Giustina and Veldhuis, 1998) and reduce GH receptor expression (King and Carter-Su, 1995). As a result, IGF-I mRNA level was thought to decrease in vivo experiment in the present study. Dexamethasone has been reported to reduce the expression in in vivo animals (Gilson et al., 2007), being consistent with our in vivo result.
In summary, GHRP-2 suppressed dexamethasone-induced Atrogin-1 mRNA expressions in in vivo rats without elevating plasma IGF-I and IGF-I mRNA in muscle. Furthermore GHRP-2 decreased dexamethasone-induced Atrogin-1 and MuRF1 expressions in C2C12 myocytes. This effect was blocked by the addition of [D-Lys3]-GHRP-6, a GHS-R-1a antagonist. These findings suggest that a direct action of GHRP-2 through GHSR-1a suppresses Atrogin-1 and MuRF1 mRNA levels in C2C12 cells. GHRP-2 might lead to the protection of muscle atrophy induced by dexamethasone.
REFERENCES
Alzghoul, M.B., Gerrard, D., Watkins, B.A., Hannon, K., 2004. Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. The FASEB Journal 18 (1), 221–223.
Baracos, V.E., Devivo, C., Hoyle, D.H., Goldberg, A.L., 1995. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. The American Journal of Physiology-Endocrinology and Metabolism 268 (5 Pt 1), E996–E1006.
Bodine, S.C., Latres, E., Baumhueter, S., Lai, V.K., Nunez, L., Clarke, B.A., Poueymirou,W.T., Panaro, F.J.,Na,E.,Dharmarajan,K., Pan, Z.Q.,Valenzuela, D.M., Dechiara, T.M., Stitt, T.N., Yancopoulos, G.D., Glass, D.J., 2001. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294 (5547), 1704–1708.
Bowers, C.Y., Granda, R., Mohan, S., Kuipers, J., Baylink, D., Veldhuis, J.D., 2004. Sustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and women. The Journal of Clinical Endocrinology and Metabolism 89 (5), 2290.2300.
Cheng, K., Chan, W.W., Barreto Jr., A., Convey, E.M., Smith, R.G., 1989. The synergistic effects of His-D-Trp-Ala-Trp-D-Phe-Lys-NH2 on growth hormone (GH)-releasing factor-stimulated GH release and intracellular adenosine 3' 5' -monophosphate accumulation in rat primary pituitary cell culture. Endocrinology 124, 2791.2798.
Day, C.S., Buranapanitkit, B., Riano, F.A., Tomaino, M.M., Somogyi, G., Sotereanos, D.G., Kuroda, R., Huard, J., 2002. Insulin growth factor-1 decreases muscle atrophy following denervation. Microsurgery 22 (4),
144.151. Falduto, M.T., Young, A.P., Hickson, R.C., 1992a. Exercise inhibits glucocorticoid- induced glutamine synthetase expression in red skeletal muscles. American Journal of Physiology-Cell Physiology 262 (1 Pt 1), C214.C220.
Falduto, M.T., Young, A.P., Hickson, R.C., 1992b. Exercise interrupts ongoing glucocorticoid-induced muscle atrophy and glutamine synthetase induction. American Journal of Physiology-Cell Physiology 263 (6 Pt 1), E1157.E1163.
Frost, R.A., Nystrom, G.J., Lang, C.H., 2003. Tumor necrosis factor-alpha decreases insulin-like growth factor-I messenger ribonucleic acid expression in C2C12 myoblasts via a Jun N-terminal kinase pathway. Endocrinology 144 (5), 1770.1779.
Gilson, H., Schakman, O., Combaret, L., Lause, P., Grobet, L., Attaix, D., Ketelslegers, J.M., Thissen, J.P., 2007. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 148 (1), 452.460.
Giustina, A., Veldhuis, J.D., 1998. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocrine Reviews 19 (6), 717.797.
Gomes, M.D., Lecker, S.H., Jagoe, R.T., Navon, A., Goldberg, A.L., 2001. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proceedings of the National Academy of Sciences of the United States of America 98 (25), 14440.14445.
Granado, M., Priego, T., Martin, A.I., Villanua, M.A., Lopez-Calderon, A., 2005. Ghrelin receptor agonist GHRP-2 prevents arthritis-induced increase in E3 ubiquitin-ligating enzymes MuRF1 and MAFbx gene expression in skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism 289 (6), E1007.E1014.
Howard, A.D., Feighner, S.D., Cully, D.F., Arena, J.P., Liberator, P.A., Rosenblum, C.I., Hamelin, M., Hreniuk, D.L., Palyha, O.C., Anderson, J., Paress, P.S., Diaz, C., Chou, M., Liu, K.K., Mckee, K.K., Pong, S.S., Chaung, L.Y., Elbrecht, A., Dashkevicz, M., Heavens, R., Rigby, M., Sirinathsinghji, D.J., Dean, D.C., Melillo, D.G., Patchett, A.A., Nargund, R., Griffin, P.R., Demartino, J.A., Gupta, S.K., Schaeffer, J.M., Smith, R.G., Van Der Ploeg, L.H., 1996. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273 (5277), 974.977.
Kanda, F., Takatani, K., Okuda, S., Matsushita, T., Chihara, K., 1999. Preventive effects of insulinlike growth factor-I on steroid-induced muscle atrophy. Muscle & Nerve 22 (2), 213.217.
Kandarian, S.C., Jackman, R.W., 2006. Intracellular signaling during skeletal muscle atrophy. Muscle & Nerve 33 (2), 155.165.
Kayali, A.G., Young, V.R., Goodman, M.N., 1987. Sensitivity of myofibrillar proteins to glucocorticoid-induced muscle proteolysis. American Journal of Physiology-Endocrinology and Metabolism 252 (5 Pt 1), E621.E626.
King, A.P., Carter-Su, C., 1995. Dexamethasone-induced antagonism of growth hormone (GH) action by down-regulation of GH binding in 3T3-F442A fibroblasts. Endocrinology 136 (11), 4796.4803.
Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H., Kangawa, K., 1999. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402 (6762), 656.660.
Lecker, S.H., Jagoe, R.T., Gilbert, A., Gomes, M., Baracos, V., Bailey, J., Price, S.R., Mitch, W.E., Goldberg, A.L., 2004. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. The FASEB journal 18 (1), 39.51.
Ma, K., Mallidis, C., Bhasin, S., Mahabadi, V., Artaza, J., Gonzalez-Cadavid, N., Arias, J., Salehian, B., 2003. Glucocorticoid-induced skeletal muscleatrophy is associated with upregulation of myostatin gene expression. American Journal of Physiology-Endocrinology and Metabolism 285 (2), E363–E371.
Mckee, K.K., Palyha, O.C., Feighner, S.D., Hreniuk, D.L., Tan, C.P., Phillips, M.S., Smith, R.G., Van Der Ploeg, L.H., Howard, A.D., 1997. Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Molecular Endocrinology 11 (4), 415–423.
Murata, M., Okimura, Y., Iida, K., Matsumoto, M., Sowa, H., Kaji, H., Kojima, M., Kangawa, K., Chihara, K., 2002. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. The Journal of Biological Chemistry 277 (7), 5667–5674.
Nijland, E.A., Strasburger, C.J., Popp-Snijders, C., Van Der Wal, P.S., Van Der Veen, E.A., 1998. A five day treatment with daily subcutaneous injections of growth hormone-releasing peptide-2 causes response attenuation and does not stimulate insulin-like growth factor-I secretion in healthy young men. European Journal of Endocrinology 139 (4), 395–401.
Papotti, M., Ghe, C., Cassoni, P., Catapano, F., Deghenghi, R., Ghigo, E., Muccioli, G., 2000. Growth hormone secretagogue binding sites in peripheral human tissues. The Journal of Clinical Endocrinology and Metabolism 85 (10), 3803–3807.
Pierno, S., De Luca, A., Desaphy, J.F., Fraysse, B., Liantonio, A., Didonna, M.P., Lograno, M., Cocchi, D., Smith, R.G., Camerino, D.C., 2003. Growth hormone secretagogues modulate the electrical and contractile properties of rat skeletal muscle through a ghrelin-specific receptor. British Journal of Pharmacology 139 (3), 575–584.
Pihoker, C., Middleton, R., Reynolds, G.A., Bowers, C.Y., Badger, T.M., 1995. Diagnostic studies with intravenous and intranasal growth hormonereleasing peptide-2 in children of short stature. The Journal of Clinical Endocrinology Metabolism 80 (10), 2987–2992.
Price, S.R., Bailey, J.L., England, B.K., 1996. Necessary but not sufficient: the role of glucocorticoids in the acidosis-induced increase in levels of mRNAs encoding proteins of the ATP-dependent proteolytic pathway in rat muscle. Mineral Electrolyte Metabolism 22 (1–3), 72–75.
Sacheck, J.M., Ohtsuka, A., Mclary, S.C., Goldberg, A.L., 2004. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. American Journal of Physiology-Endocrinology and Metabolism 287 (4), E591.E601.
Sawada, H., Sugihara, H., Onose, H., Minami, S., Shibasaki, T., Wakabayashi, I., 1994. Effect of D-Ala-D-beta Nal-Ala-Trp-D-Phe-Lys-NH2 (KP-102) on GH secretion in urethan-anesthetized rats. Regulatory Peptides 53 (3), 195.201.
Schakman, O., Gilson, H., De Coninck, V., Lause, P., Verniers, J., Havaux, X., Ketelslegers, J.M., Thissen, J.P., 2005. Insulin-like growth factor-I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid- treated rats. Endocrinology 146 (4), 1789.1797.
Smith, R.G., Cheng, K., Schoen,W.R., Pong, S.S., Hickey, G., Jacks, T., Butler, B., Chan, W.W., Chaung, L.Y., Judith, F., 1993. A nonpeptidyl growth hormone secretagogue. Science 260 (5114), 1640.1643.
Stitt, T.N., Drujan, D., Clarke, B.A., Panaro, F., Timofeyva, Y., Kline, W.O., Gonzalez, M., Yancopoulos, G.D., Glass, D.J., 2004. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Molecular Cell 14 (3), 395.403.
Tiao, G., Hobler, S., Wang, J.J., Meyer, T.A., Luchette, F.A., Fischer, J.E., Hasselgren, P.O., 1997. Sepsis is associated with increased mRNAs of the ubiquitin-proteasome proteolytic pathway in human skeletal muscle. Journal of Clinical Investigation 99 (2), 163.168.
Tischler, M.E., Rosenberg, S., Satarug, S., Henriksen, E.J., Kirby, C.R., Tome, M., Chase, P., 1990. Different mechanisms of increased proteolysis in atrophy induced by denervation or unweighting of rat soleus muscle. Metabolism 39 (7), 756.763.
Tschop, M., Statnick, M.A., Suter, T.M., Heiman, M.L., 2002. GH-releasing peptide-2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology 143 (2), 558.568.
Wu, D., Chen, C., Zhang, J., Bowers, C.Y., Clarke, I.J., 1996. The effects of GH-releasing peptide-6 (GHRP-6) and GHRP-2 on intracellular adenosine 3' 5' -monophosphate (cAMP) levels and GH secretion in ovine and rat somatotrophs. Journal of Endocrinology 148 (2), 197.205.
Last edited:
- Joined
- Jul 25, 2008
- Messages
- 1,700
GHRPs Anabolic
As always I highlighted in red so if you want you can just read the red parts and get a gist of what the study found.
ANABOLISM -> Ghrelin, Des-Acyl Ghrelin, GHRP-2, GHRP-6, Hexarelin = Promote Differention & Fusion of Muscle Cells <- ?
As always I highlighted in red so if you want you can just read the red parts and get a gist of what the study found.
ANABOLISM -> Ghrelin, Des-Acyl Ghrelin, GHRP-2, GHRP-6, Hexarelin = Promote Differention & Fusion of Muscle Cells <- ?
Ghrelin and Des-Acyl Ghrelin Promote Differentiation and Fusion of C2C12 Skeletal Muscle Cells, Nicoletta Filigheddu, Molecular Biology of the Cell Vol. 18, 986–994, March 2007
...
DISCUSSION
Skeletal muscle satellite cells are quiescent mononucleated myoblasts, located between the sarcolemma and the basal membrane of terminally differentiated adult muscle fibers. On muscle diseases or direct injury, quiescent satellite cells are activated to undergo proliferation and eventually differentiate to allow muscle regeneration.
Skeletal muscle regeneration involves, sequentially, satellite cell proliferation, commitment to terminal differentiation, cell fusion into multinucleated syncitia, and muscle fiber formation.
Such mechanisms leading to muscle regeneration are poorly understood; they seem to recapitulate the embryonic program of differentiation, although the extracellular factors regulating such processes may be different.
Satellite cell differentiation into skeletal muscle can be subdivided into temporally separable events, coordinated by the expression of proteins of the muscle regulatory factors family, such as myogenin, and of cyclin-dependent kinase inhibitor of the p21 family (Andres and Walsh, 1996), resulting in cell cycle exit and commitment to terminal differentiation. Later on, expression of muscle contractile proteins, such as MHCs and myosin light chains (MLCs), are hallmarks of phenotypic differentiation. Finally, fusion of myocytes into multinucleated myotubes is the terminal step of muscle differentiation.
The growing interest in skeletal muscle regeneration is associated to the opening of new therapeutic strategies for several muscular degenerative pathologies such as dystrophies, muscular atrophy, and cachexia associated to aging, cancer, chronic heart failure, and acquired immunodeficiency syndrome as well as the treatments of skeletal muscle injury after trauma.
Although Ghrelin (GHR) is a circulating hormone mainly secreted by the stomach, it is also synthesized in a number of tissues, suggesting both endocrine and paracrine effects (Gnanapavan et al., 2002).
The evidence that 1) Ghrelin (GHR) up-regulation is specifically associated to either congestive heart failure (CHF)- or cancer- induced cachexia (Nagaya et al., 2001, Shimizu et al., 2003) and that its administration strongly prevents CHF associated cachexia (Nagaya et al., 2004); 2) GHR, (Des-Acyl Ghrelin) D-GHR, and Growth Hormone Secretagogues (GHSs) inhibit apoptosis of cardiac myocytes (Filigheddu et al., 2001; Baldanzi et al., 2002); and 3) skeletal muscle features high binding sites for synthetic GHSs (Papotti et al., 2000), lead us to speculate that GHR and D-GHR may act directly also on skeletal muscle. Indeed, we observed that both GHR and D-GHR stimulate tyrosine phosphorylation of several proteins and activate ERK-1/2 and Akt (data not shown), indicating that both factors could exert a biological activity on these cells.
Here, we show that nanomolar concentrations of both GHR and D-GHR induce the differentiation of proliferating skeletal myoblasts in a concentration-dependent manner and promote their fusion into multinucleated syncitia in vitro. The cellular and molecular mechanisms by which GHR and D-GHR elicit these responses are not known. Cell cycle withdrawal is a prerequisite for myogenic terminal differentiation (Walsh and Perlman, 1997). Indeed, the ability of GHR and D-GHR to reduce DNA synthesis of proliferating C2C12 myoblasts is highly consistent with their prodifferentiative activity. However, inhibition of cell proliferation is not sufficient to elicit muscle differentiation. For example, myostatin inhibits both proliferation and differentiation of C2C12 myoblasts, through down-regulation of MyoD and myogenin expression (Joulia et al., 2003). Conversely, GHR and D-GHR, beyond inhibiting cell proliferation, induce the expression of myogenin, which is required for the complete program of differentiation of skeletal myoblasts to proceed (Zhang et al., 1999). To our knowledge this is the first evidence for an extracellular factor able to induce muscle differentiation of proliferating skeletal myoblasts in GM.
In proliferating C2C12 myoblasts, activation of p38 pathway obtained by overexpression of constitutively active MKK6 is sufficient to induce myogenin expression, cell cycle exit, and skeletal muscle terminal differentiation (Wu et al., 2000). Thus, we investigated whether GHR and D-GHR prodifferentiative activity is mediated by p38. Consistently, inhibition of p38 by cell treatment with SB203580 resulted in the partial albeit significant inhibition of GHR and D-GHR induced differentiative activity. In addition, we also showed that both GHR and D-GHR activate p38. Altogether, these data demonstrate that GHR and D-GHR act as antiproliferative and prodifferentiative factors by stimulating the p38 pathway.
The lack of expression of Growth Hormone Secretagogue Receptor One-A (GHSR-1a) in either C2C12 myoblasts and skeletal muscle tissue (Gnanapavan et al., 2002) as well as the activity exerted by D-GHR suggest that GHR and D-GHR–differentiating activities are mediated by a yet unidentified receptor, common to both acylated and unacylated peptide and distinct from GHSR-1a. Indeed, here we showed that C2C12 cells feature high-affinity common binding sites for both GHR and D-GHR. Such binding sites are specific, because they do not recognize either N-terminal truncated ghrelin or motilin, which are unable to induce differentiation. These studies also demonstrate that the N-terminal portion of the GHR peptide is required for binding and induction of C2C12 muscular differentiation. Together, these data provide further evidence for novel GHR receptor subtypes, which do not discriminate between the acylated and unacylated peptide. Although evidence for common GHR and D-GHR receptors have been reported in several cells, including a cardiomyocyte-derived cell line (Baldanzi et al., 2002), this is the first evidence for their expression in skeletal muscle.
We also verified whether the ghrelin gene is up-regulated in C2C12 myoblasts induced to differentiate in DM. However, no difference of ghrelin expression was detected by real-time RT-PCR between proliferating and differentiating cells (data not shown), suggesting that GHR gene product is not involved in DM-induced skeletal muscle differentiation in vitro.
By showing that GHR and D-GHR stimulate terminal differentiation of skeletal myoblasts in vitro, we may raise the hypothesis that the function of GHR gene may be involved in skeletal muscle differentiation in vivo. However, the lack of a consistent phenotype in GHR knockout mice, suggests that GHR function is not required for myogenesis during development. Consistently, we have not detected any GHR expression in somites or related structures during embryonic development by in situ hybridization (data not shown). However, although not essential for embryo development, GHR might be involved in the complex process of myogenesis in the adulthood, i.e., in regenerative processes of skeletal muscle. This hypothesis is consistent with the data showing that FGF6 is not required for muscle development, but is required in the adult for damage-induced muscle regeneration (Floss et al., 1997).
Upon muscular injury, skeletal myoblasts are activated to terminally differentiate through an autocrine/paracrine loop. We may speculate that GHR would contribute to skeletal muscle plasticity, promoting the differentiation and fusion of myoblasts in the damaged muscles. If this hypothesis would be proved, the activation of the receptor mediating GHR and D-GHR differentiative activity as well as the over-expression of the hormone may provide novel therapeutic strategies for the reduction or retardation of several skeletalmuscle pathologies, including dystrophies, atrophies, and cachexia.
REFERENCES
Amendola, M., Venneri, M. A., Biffi, A., Vigna, E., and Naldini, L. (2005). Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat. Biotechnol. 23, 108–116.
Andreis, P. G., Malendowicz, L. K., Trejter, M., Neri, G., Spinazzi, R., Rossi, G. P., and Nussdorfer, G. G. (2003). Ghrelin and growth hormone secretagogue receptor are expressed in the rat adrenal cortex: evidence that ghrelin stimulates the growth, but not the secretory activity of adrenal cells. FEBS Lett. 536, 173–179.
Andres, V., and Walsh, K. (1996). Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J. Cell Biol. 132, 657–666.
Baldanzi, G. et al. (2002). Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/ AKT. J. Cell Biol. 159, 1029–1037.
Barreiro, M. L., Gaytan, F., Castellano, J. M., Suominen, J. S., Roa, J., Gaytan, M., Aguilar, E., Dieguez, C., Toppari, J., and Tena-Sempere, M. (2004). Ghrelin inhibits the proliferative activity of immature Leydig cells in vivo and regulates stem cell factor messenger ribonucleic acid expression in rat testis. Endocrinology 145, 4825–4834.
Blau, H. M., Pavlath, G. K., Hardeman, E. C., Chiu, C. P., Silberstein, L., Webster, S. G., Miller, S. C., and Webster, C. (1985). Plasticity of the differentiated state. Science 230, 758–766.
Cassoni, P., Ghe`, C., Marrocco, T., Tarabra, E., Allia, E., Catapano, F., Deghenghi, R., Ghigo, E., Papotti, M., and Muccioli, G. (2004). Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. Eur. J. Endocrinol. 150, 173–184.
Cassoni, P., Papotti, M., Ghe, C., Catapano, F., Sapino, A., Graziani, A., Deghenghi, R., Reissmann, T., Ghigo, E., and Muccioli, G. (2001). Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J. Clin. Endocrinol. Metab. 86, 1738–1745.
Choi, K., Roh, S. G., Hong, Y. H., Shrestha, Y. B., Hishikawa, D., Chen, C., Kojima, M., Kangawa, K., and Sasaki, S. (2003). The role of ghrelin and growth hormone secretagogues receptor on rat adipogenesis. Endocrinology 144, 754–759.
De Vriese, C., Gregoire, F., De Neef, P., Robberecht, P., and Delporte, C. (2005). Ghrelin is produced by the human erythroleukemic HEL cell line and involved in an autocrine pathway leading to cell proliferation. Endocrinology 146, 1514–1522.
Delhanty, P. J., van der Eerden, B. C., van der Velde, M., Gauna, C., Pols, H. A., Jahr, H., Chiba, H., van der Lely, A. J., and van Leeuwen, J. P. (2006). Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen- activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J. Endocrinol. 188, 37–47.
Duxbury, M. S., Waseem, T., Ito, H., Robinson, M. K., Zinner, M. J., Ashley, S. W., and Whang, E. E. (2003). Ghrelin promotes pancreatic adenocarcinoma cellular proliferation and invasiveness. Biochem. Biophys. Res. Commun. 309, 464–468.
Filigheddu N. et al. (2001). Hexarelin protects H9C2 cardiomyocytes from doxorubicin-induced cell death. Endocrine 14, 113–119.
Floss, T., Arnold, H. H., and Braun, T. (1997). A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 11, 2040–2051.
Fukushima N. et al. (2005). Ghrelin directly regulates bone formation. J. Bone Miner. Res. 20, 790–798.
Ghe, C., Cassoni, P., Catapano, F., Marrocco, T., Deghenghi, R., Ghigo, E., Muccioli, G., and Papotti, M. (2002). The antiproliferative effect of syntheticpeptidyl GH secretagogues in human CALU-1 lung carcinoma cells. Endocrinology 143, 484–491.
Gnanapavan, S., Kola, B., Bustin, S. A., Morris, D. G., McGee, P., Fairclough, P., Bhattacharya, S., Carpenter, R., Grossman, A. B., and Korbonits, M. (2002). The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 87, 2988–2991.
Granado, M., Priego, T., Martin, A. I., Villanua, M. A., and Lopez-Calderon, A. (2005). Ghrelin receptor agonist GHRP-2 prevents arthritis-induced increase in E3 ubiquitin-ligating enzymes MuRF1 and MAFbx gene expression in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 289, E1007–E1014.
Groschl, M., Topf, H. G., Bohlender, J., Zenk, J., Klussmann, S., Dotsch, J., Rascher, W., and Rauh, M. (2005). Identification of ghrelin in human saliva: production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin. Chem. 51, 1–10.
Howard H. D. et al. (1996). A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273, 974–976.
Jeffery, P. L., Herington, A. C., and Chopin, L. K. (2002). Expression and action of the growth hormone releasing peptide ghrelin and its receptor in prostate cancer cell lines. J. Endocrinol. 172, R7–R11.
Joulia, D., Bernardi, H., Garandel, V., Rabenoelina, F., Vernus, B., and Cabello, G. (2003). Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp. Cell Res. 286, 263–275.
Kim M. S. et al. (2004). The mitogenic and antiapoptotic actions of ghrelin in 3T3–L1 adipocytes. Mol. Endocrinol. 18, 2291–2301.
Kim, S. W., Her, S. J., Park, S. J., Kim, D., Park, K. S., Lee, H. K., Han, B. H., Kim, M. S., Shin, C. S., and Kim, S. Y. (2005). Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3–E1 cells. Bone 37, 359–369.
Kohno, D., Gao, H. Z., Muroya, S., Kikuyama, S., and Yada, T. (2003). Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca(2) signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes 52, 948–956.
Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H., and Kangawa, K. (1999). Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660.
Maccarinelli, G., Sibilia, V., Torsello, A., Raimondo, F., Pitto, M., Giustina, A., Netti, C., and Cocchi, D. (2005). Ghrelin regulates proliferation and differentiation of osteoblastic cells. J. Endocrinol. 184, 249–256.
Mazzocchi, G., Neri, G., Rucinski, M., Rebuffat, P., Spinazzi, R., Malendowicz, L. K., and Nussdorfer, G. G. (2004). Ghrelin enhances the growth of cultured human adrenal zona glomerulosa cells by exerting MAPK-mediated proliferogenic and antiapoptotic effects. Peptides 25, 1269–1277.
Muccioli, G., Papotti, M., Locatelli, V., Ghigo, E., and Deghenghi, R. (2001). Binding of 125I-labelled ghrelin to membranes from human hypothalamus and pituitary gland. J. Endocrinol. Invest. 24, RC7–RC9.
Muccioli, G., Pons, N., Ghe`, C., Catapano, F., Granata, R., and Ghigo, E. (2004). Ghrelin and des-acyl ghrelin both inhibit isoproterenol-induced lipolysis in rat adipocytes via a non-type growth hormone secretagogue receptor. Eur. J. Pharmacol. 498, 27–35.
Nagaya, N., Itoh, T., Murakami, S., Oya, H., Uematsu, M., Miyatake, K., and Kangawa, K. (2005). Treatment of cachexia with ghrelin in patients with COPD. Chest 128, 1187–1193.
Nagaya, N., Moriya, J., Yasumura, Y., Uematsu, M., Ono, F., Shimizu, W., Ueno, K., Kitakaze, M., Miyatake, K., and Kangawa, K. (2004). Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation 110, 3674– 3679.
Nagaya N. et al. (2001). Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/ catabolic factors. Circulation 104, 2034–2038.
Nanzer, A. M., Khalaf, S., Mozid, A. M., Fowkes, R. C., Patel, M. V., Burrin, J. M., Grossman, A. B., and Korbonits, M. (2004). Ghrelin exerts a proliferative effect on a rat pituitary somatotroph cell line via the mitogen-activated protein kinase pathway. Eur. J. Endocrinol. 151, 233–240.
Papotti, M., Ghe`, C., Cassoni, P., Catapano, F., Deghenghi, R., Ghigo, E., and Muccioli, G. (2000). Growth hormone secretagogue. GHS binding sites in peripheral human tissues. J. Clin. Endocrinol. Metab. 85, 3803–3807.
Pettersson, I., Muccioli, G., Granata, R., Deghenghi, R., Ghigo, E., Ohlsson, C., and Isgaard, J. (2002). Natural (ghrelin) and synthetic (hexarelin) GH secretagogues stimulate H9c2 cardiomyocyte cell proliferation. J. Endocrinol. 175, 201–209.
Reimer, M. K., Pacini, G., and Ahren, B. (2003). Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology 144, 916–921.
Shimizu, Y., Nagaya, N., Isobe, T., Imazu, M., Okumura, H., Hosoda, H., Kojima, M., Kangawa, K., and Kohno, N. (2003). Increased plasma ghrelin level in lung cancer cachexia. Clin. Cancer Res. 9, 774–778.
Tesauro, M., Schinzari, F., Iantorno, M., Rizza, S., Melina, D., Lauro, D., and Cardillo, C. (2005). Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation 112, 2986–2992.
Volante, M., Allia, E., Fulcheri, E., Cassoni, P., Ghigo, E., Muccioli, G., and Papotti, M. (2003). Ghrelin in fetal thyroid and follicular tumors and cell lines: expression and effects on tumor growth. Am. J. Pathol. 162, 645–654.
Walsh, K., and Perlman, H. (1997). Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev. 7, 597–602.
Wu, Z., Woodring, P. J., Bhakta, K. S., Tamura, K., Wen, F., Feramisco, J. R., Karin, M., Wang, J. Y., and Puri, P. L. (2000). p38 and extracellular signal regulatedkinases regulate the myogenic program at multiple steps. Mol. Cell Biol. 20, 3951–3964.
Xia, Q., Pang, W., Pan, H., Zheng, Y., Kang, J. S., and Zhu, S. G. (2004). Effects of ghrelin on the proliferation and secretion of splenic T lymphocytes in mice. Regul. Pept. 122, 173–178.
Zhang, P., Wong, C., Liu, D., Finegold, M., Harper, J. W., and Elledge, S. J. (1999). p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 13, 213–224.
Zhang, W., Hu, Y., Lin, T. R., Fan, Y., and Mulholland, M. W. (2005). Stimulation of neurogenesis in rat nucleus of the solitary tract by ghrelin. Peptides 26, 2280–2288.
Zhang, W., Zhao, L., Lin, T. R., Chai, B., Fan, Y., Gantz, I., and Mulholland, M. W. (2004). Inhibition of adipogenesis by ghrelin. Mol. Biol. Cell 15, 2484– 2491
...
DISCUSSION
Skeletal muscle satellite cells are quiescent mononucleated myoblasts, located between the sarcolemma and the basal membrane of terminally differentiated adult muscle fibers. On muscle diseases or direct injury, quiescent satellite cells are activated to undergo proliferation and eventually differentiate to allow muscle regeneration.
Skeletal muscle regeneration involves, sequentially, satellite cell proliferation, commitment to terminal differentiation, cell fusion into multinucleated syncitia, and muscle fiber formation.
Such mechanisms leading to muscle regeneration are poorly understood; they seem to recapitulate the embryonic program of differentiation, although the extracellular factors regulating such processes may be different.
Satellite cell differentiation into skeletal muscle can be subdivided into temporally separable events, coordinated by the expression of proteins of the muscle regulatory factors family, such as myogenin, and of cyclin-dependent kinase inhibitor of the p21 family (Andres and Walsh, 1996), resulting in cell cycle exit and commitment to terminal differentiation. Later on, expression of muscle contractile proteins, such as MHCs and myosin light chains (MLCs), are hallmarks of phenotypic differentiation. Finally, fusion of myocytes into multinucleated myotubes is the terminal step of muscle differentiation.
The growing interest in skeletal muscle regeneration is associated to the opening of new therapeutic strategies for several muscular degenerative pathologies such as dystrophies, muscular atrophy, and cachexia associated to aging, cancer, chronic heart failure, and acquired immunodeficiency syndrome as well as the treatments of skeletal muscle injury after trauma.
Although Ghrelin (GHR) is a circulating hormone mainly secreted by the stomach, it is also synthesized in a number of tissues, suggesting both endocrine and paracrine effects (Gnanapavan et al., 2002).
The evidence that 1) Ghrelin (GHR) up-regulation is specifically associated to either congestive heart failure (CHF)- or cancer- induced cachexia (Nagaya et al., 2001, Shimizu et al., 2003) and that its administration strongly prevents CHF associated cachexia (Nagaya et al., 2004); 2) GHR, (Des-Acyl Ghrelin) D-GHR, and Growth Hormone Secretagogues (GHSs) inhibit apoptosis of cardiac myocytes (Filigheddu et al., 2001; Baldanzi et al., 2002); and 3) skeletal muscle features high binding sites for synthetic GHSs (Papotti et al., 2000), lead us to speculate that GHR and D-GHR may act directly also on skeletal muscle. Indeed, we observed that both GHR and D-GHR stimulate tyrosine phosphorylation of several proteins and activate ERK-1/2 and Akt (data not shown), indicating that both factors could exert a biological activity on these cells.
Here, we show that nanomolar concentrations of both GHR and D-GHR induce the differentiation of proliferating skeletal myoblasts in a concentration-dependent manner and promote their fusion into multinucleated syncitia in vitro. The cellular and molecular mechanisms by which GHR and D-GHR elicit these responses are not known. Cell cycle withdrawal is a prerequisite for myogenic terminal differentiation (Walsh and Perlman, 1997). Indeed, the ability of GHR and D-GHR to reduce DNA synthesis of proliferating C2C12 myoblasts is highly consistent with their prodifferentiative activity. However, inhibition of cell proliferation is not sufficient to elicit muscle differentiation. For example, myostatin inhibits both proliferation and differentiation of C2C12 myoblasts, through down-regulation of MyoD and myogenin expression (Joulia et al., 2003). Conversely, GHR and D-GHR, beyond inhibiting cell proliferation, induce the expression of myogenin, which is required for the complete program of differentiation of skeletal myoblasts to proceed (Zhang et al., 1999). To our knowledge this is the first evidence for an extracellular factor able to induce muscle differentiation of proliferating skeletal myoblasts in GM.
In proliferating C2C12 myoblasts, activation of p38 pathway obtained by overexpression of constitutively active MKK6 is sufficient to induce myogenin expression, cell cycle exit, and skeletal muscle terminal differentiation (Wu et al., 2000). Thus, we investigated whether GHR and D-GHR prodifferentiative activity is mediated by p38. Consistently, inhibition of p38 by cell treatment with SB203580 resulted in the partial albeit significant inhibition of GHR and D-GHR induced differentiative activity. In addition, we also showed that both GHR and D-GHR activate p38. Altogether, these data demonstrate that GHR and D-GHR act as antiproliferative and prodifferentiative factors by stimulating the p38 pathway.
The lack of expression of Growth Hormone Secretagogue Receptor One-A (GHSR-1a) in either C2C12 myoblasts and skeletal muscle tissue (Gnanapavan et al., 2002) as well as the activity exerted by D-GHR suggest that GHR and D-GHR–differentiating activities are mediated by a yet unidentified receptor, common to both acylated and unacylated peptide and distinct from GHSR-1a. Indeed, here we showed that C2C12 cells feature high-affinity common binding sites for both GHR and D-GHR. Such binding sites are specific, because they do not recognize either N-terminal truncated ghrelin or motilin, which are unable to induce differentiation. These studies also demonstrate that the N-terminal portion of the GHR peptide is required for binding and induction of C2C12 muscular differentiation. Together, these data provide further evidence for novel GHR receptor subtypes, which do not discriminate between the acylated and unacylated peptide. Although evidence for common GHR and D-GHR receptors have been reported in several cells, including a cardiomyocyte-derived cell line (Baldanzi et al., 2002), this is the first evidence for their expression in skeletal muscle.
We also verified whether the ghrelin gene is up-regulated in C2C12 myoblasts induced to differentiate in DM. However, no difference of ghrelin expression was detected by real-time RT-PCR between proliferating and differentiating cells (data not shown), suggesting that GHR gene product is not involved in DM-induced skeletal muscle differentiation in vitro.
By showing that GHR and D-GHR stimulate terminal differentiation of skeletal myoblasts in vitro, we may raise the hypothesis that the function of GHR gene may be involved in skeletal muscle differentiation in vivo. However, the lack of a consistent phenotype in GHR knockout mice, suggests that GHR function is not required for myogenesis during development. Consistently, we have not detected any GHR expression in somites or related structures during embryonic development by in situ hybridization (data not shown). However, although not essential for embryo development, GHR might be involved in the complex process of myogenesis in the adulthood, i.e., in regenerative processes of skeletal muscle. This hypothesis is consistent with the data showing that FGF6 is not required for muscle development, but is required in the adult for damage-induced muscle regeneration (Floss et al., 1997).
Upon muscular injury, skeletal myoblasts are activated to terminally differentiate through an autocrine/paracrine loop. We may speculate that GHR would contribute to skeletal muscle plasticity, promoting the differentiation and fusion of myoblasts in the damaged muscles. If this hypothesis would be proved, the activation of the receptor mediating GHR and D-GHR differentiative activity as well as the over-expression of the hormone may provide novel therapeutic strategies for the reduction or retardation of several skeletalmuscle pathologies, including dystrophies, atrophies, and cachexia.
REFERENCES
Amendola, M., Venneri, M. A., Biffi, A., Vigna, E., and Naldini, L. (2005). Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat. Biotechnol. 23, 108–116.
Andreis, P. G., Malendowicz, L. K., Trejter, M., Neri, G., Spinazzi, R., Rossi, G. P., and Nussdorfer, G. G. (2003). Ghrelin and growth hormone secretagogue receptor are expressed in the rat adrenal cortex: evidence that ghrelin stimulates the growth, but not the secretory activity of adrenal cells. FEBS Lett. 536, 173–179.
Andres, V., and Walsh, K. (1996). Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J. Cell Biol. 132, 657–666.
Baldanzi, G. et al. (2002). Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/ AKT. J. Cell Biol. 159, 1029–1037.
Barreiro, M. L., Gaytan, F., Castellano, J. M., Suominen, J. S., Roa, J., Gaytan, M., Aguilar, E., Dieguez, C., Toppari, J., and Tena-Sempere, M. (2004). Ghrelin inhibits the proliferative activity of immature Leydig cells in vivo and regulates stem cell factor messenger ribonucleic acid expression in rat testis. Endocrinology 145, 4825–4834.
Blau, H. M., Pavlath, G. K., Hardeman, E. C., Chiu, C. P., Silberstein, L., Webster, S. G., Miller, S. C., and Webster, C. (1985). Plasticity of the differentiated state. Science 230, 758–766.
Cassoni, P., Ghe`, C., Marrocco, T., Tarabra, E., Allia, E., Catapano, F., Deghenghi, R., Ghigo, E., Papotti, M., and Muccioli, G. (2004). Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. Eur. J. Endocrinol. 150, 173–184.
Cassoni, P., Papotti, M., Ghe, C., Catapano, F., Sapino, A., Graziani, A., Deghenghi, R., Reissmann, T., Ghigo, E., and Muccioli, G. (2001). Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J. Clin. Endocrinol. Metab. 86, 1738–1745.
Choi, K., Roh, S. G., Hong, Y. H., Shrestha, Y. B., Hishikawa, D., Chen, C., Kojima, M., Kangawa, K., and Sasaki, S. (2003). The role of ghrelin and growth hormone secretagogues receptor on rat adipogenesis. Endocrinology 144, 754–759.
De Vriese, C., Gregoire, F., De Neef, P., Robberecht, P., and Delporte, C. (2005). Ghrelin is produced by the human erythroleukemic HEL cell line and involved in an autocrine pathway leading to cell proliferation. Endocrinology 146, 1514–1522.
Delhanty, P. J., van der Eerden, B. C., van der Velde, M., Gauna, C., Pols, H. A., Jahr, H., Chiba, H., van der Lely, A. J., and van Leeuwen, J. P. (2006). Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen- activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J. Endocrinol. 188, 37–47.
Duxbury, M. S., Waseem, T., Ito, H., Robinson, M. K., Zinner, M. J., Ashley, S. W., and Whang, E. E. (2003). Ghrelin promotes pancreatic adenocarcinoma cellular proliferation and invasiveness. Biochem. Biophys. Res. Commun. 309, 464–468.
Filigheddu N. et al. (2001). Hexarelin protects H9C2 cardiomyocytes from doxorubicin-induced cell death. Endocrine 14, 113–119.
Floss, T., Arnold, H. H., and Braun, T. (1997). A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 11, 2040–2051.
Fukushima N. et al. (2005). Ghrelin directly regulates bone formation. J. Bone Miner. Res. 20, 790–798.
Ghe, C., Cassoni, P., Catapano, F., Marrocco, T., Deghenghi, R., Ghigo, E., Muccioli, G., and Papotti, M. (2002). The antiproliferative effect of syntheticpeptidyl GH secretagogues in human CALU-1 lung carcinoma cells. Endocrinology 143, 484–491.
Gnanapavan, S., Kola, B., Bustin, S. A., Morris, D. G., McGee, P., Fairclough, P., Bhattacharya, S., Carpenter, R., Grossman, A. B., and Korbonits, M. (2002). The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 87, 2988–2991.
Granado, M., Priego, T., Martin, A. I., Villanua, M. A., and Lopez-Calderon, A. (2005). Ghrelin receptor agonist GHRP-2 prevents arthritis-induced increase in E3 ubiquitin-ligating enzymes MuRF1 and MAFbx gene expression in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 289, E1007–E1014.
Groschl, M., Topf, H. G., Bohlender, J., Zenk, J., Klussmann, S., Dotsch, J., Rascher, W., and Rauh, M. (2005). Identification of ghrelin in human saliva: production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin. Chem. 51, 1–10.
Howard H. D. et al. (1996). A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273, 974–976.
Jeffery, P. L., Herington, A. C., and Chopin, L. K. (2002). Expression and action of the growth hormone releasing peptide ghrelin and its receptor in prostate cancer cell lines. J. Endocrinol. 172, R7–R11.
Joulia, D., Bernardi, H., Garandel, V., Rabenoelina, F., Vernus, B., and Cabello, G. (2003). Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp. Cell Res. 286, 263–275.
Kim M. S. et al. (2004). The mitogenic and antiapoptotic actions of ghrelin in 3T3–L1 adipocytes. Mol. Endocrinol. 18, 2291–2301.
Kim, S. W., Her, S. J., Park, S. J., Kim, D., Park, K. S., Lee, H. K., Han, B. H., Kim, M. S., Shin, C. S., and Kim, S. Y. (2005). Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3–E1 cells. Bone 37, 359–369.
Kohno, D., Gao, H. Z., Muroya, S., Kikuyama, S., and Yada, T. (2003). Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca(2) signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes 52, 948–956.
Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H., and Kangawa, K. (1999). Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660.
Maccarinelli, G., Sibilia, V., Torsello, A., Raimondo, F., Pitto, M., Giustina, A., Netti, C., and Cocchi, D. (2005). Ghrelin regulates proliferation and differentiation of osteoblastic cells. J. Endocrinol. 184, 249–256.
Mazzocchi, G., Neri, G., Rucinski, M., Rebuffat, P., Spinazzi, R., Malendowicz, L. K., and Nussdorfer, G. G. (2004). Ghrelin enhances the growth of cultured human adrenal zona glomerulosa cells by exerting MAPK-mediated proliferogenic and antiapoptotic effects. Peptides 25, 1269–1277.
Muccioli, G., Papotti, M., Locatelli, V., Ghigo, E., and Deghenghi, R. (2001). Binding of 125I-labelled ghrelin to membranes from human hypothalamus and pituitary gland. J. Endocrinol. Invest. 24, RC7–RC9.
Muccioli, G., Pons, N., Ghe`, C., Catapano, F., Granata, R., and Ghigo, E. (2004). Ghrelin and des-acyl ghrelin both inhibit isoproterenol-induced lipolysis in rat adipocytes via a non-type growth hormone secretagogue receptor. Eur. J. Pharmacol. 498, 27–35.
Nagaya, N., Itoh, T., Murakami, S., Oya, H., Uematsu, M., Miyatake, K., and Kangawa, K. (2005). Treatment of cachexia with ghrelin in patients with COPD. Chest 128, 1187–1193.
Nagaya, N., Moriya, J., Yasumura, Y., Uematsu, M., Ono, F., Shimizu, W., Ueno, K., Kitakaze, M., Miyatake, K., and Kangawa, K. (2004). Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation 110, 3674– 3679.
Nagaya N. et al. (2001). Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/ catabolic factors. Circulation 104, 2034–2038.
Nanzer, A. M., Khalaf, S., Mozid, A. M., Fowkes, R. C., Patel, M. V., Burrin, J. M., Grossman, A. B., and Korbonits, M. (2004). Ghrelin exerts a proliferative effect on a rat pituitary somatotroph cell line via the mitogen-activated protein kinase pathway. Eur. J. Endocrinol. 151, 233–240.
Papotti, M., Ghe`, C., Cassoni, P., Catapano, F., Deghenghi, R., Ghigo, E., and Muccioli, G. (2000). Growth hormone secretagogue. GHS binding sites in peripheral human tissues. J. Clin. Endocrinol. Metab. 85, 3803–3807.
Pettersson, I., Muccioli, G., Granata, R., Deghenghi, R., Ghigo, E., Ohlsson, C., and Isgaard, J. (2002). Natural (ghrelin) and synthetic (hexarelin) GH secretagogues stimulate H9c2 cardiomyocyte cell proliferation. J. Endocrinol. 175, 201–209.
Reimer, M. K., Pacini, G., and Ahren, B. (2003). Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology 144, 916–921.
Shimizu, Y., Nagaya, N., Isobe, T., Imazu, M., Okumura, H., Hosoda, H., Kojima, M., Kangawa, K., and Kohno, N. (2003). Increased plasma ghrelin level in lung cancer cachexia. Clin. Cancer Res. 9, 774–778.
Tesauro, M., Schinzari, F., Iantorno, M., Rizza, S., Melina, D., Lauro, D., and Cardillo, C. (2005). Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation 112, 2986–2992.
Volante, M., Allia, E., Fulcheri, E., Cassoni, P., Ghigo, E., Muccioli, G., and Papotti, M. (2003). Ghrelin in fetal thyroid and follicular tumors and cell lines: expression and effects on tumor growth. Am. J. Pathol. 162, 645–654.
Walsh, K., and Perlman, H. (1997). Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev. 7, 597–602.
Wu, Z., Woodring, P. J., Bhakta, K. S., Tamura, K., Wen, F., Feramisco, J. R., Karin, M., Wang, J. Y., and Puri, P. L. (2000). p38 and extracellular signal regulatedkinases regulate the myogenic program at multiple steps. Mol. Cell Biol. 20, 3951–3964.
Xia, Q., Pang, W., Pan, H., Zheng, Y., Kang, J. S., and Zhu, S. G. (2004). Effects of ghrelin on the proliferation and secretion of splenic T lymphocytes in mice. Regul. Pept. 122, 173–178.
Zhang, P., Wong, C., Liu, D., Finegold, M., Harper, J. W., and Elledge, S. J. (1999). p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 13, 213–224.
Zhang, W., Hu, Y., Lin, T. R., Fan, Y., and Mulholland, M. W. (2005). Stimulation of neurogenesis in rat nucleus of the solitary tract by ghrelin. Peptides 26, 2280–2288.
Zhang, W., Zhao, L., Lin, T. R., Chai, B., Fan, Y., Gantz, I., and Mulholland, M. W. (2004). Inhibition of adipogenesis by ghrelin. Mol. Biol. Cell 15, 2484– 2491
- Joined
- Jul 25, 2008
- Messages
- 1,700
Note to self
Dat's Temporary Notepad [1//19/2009]
Note #1:
Note: #2
NOT FOR PUBLIC VIEWING
Dat's Temporary Notepad [1//19/2009]
Note #1:
Pull following study. Followup on Fibroblast Growth Factor (particularly 6) as a replacement agent for Mechano Growth Factor (MGF) in the promotion of proliferation.
Note: Unlike MGF there is a receptor for Fibroblast Growth Factor.
"FGF6 is not required for muscle development, but is required in the adult for damage-induced muscle regeneration (Floss et al., 1997)." - Floss, T., Arnold, H. H., and Braun, T. (1997). A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 11, 2040–2051.
Note: Unlike MGF there is a receptor for Fibroblast Growth Factor.
Note: #2
NOT FOR PUBLIC VIEWING
Last edited:
Similar threads
Popular tags
aas
aas testing
anabolic steroids
anabolics online
anabolid steroids
anadrol
anadrol drol tabs inj
anavar
anavar and winnie
body building
body building supplements
bodybuilder
bodybuilding
bodybuilding steroid test
clenbuterol
cycle
deca tren dosage
deca-durobolin
dianabol
dianabol and oxy
dragon pharma
gear
hcg
hgh
motivation
muscle building
muscle mass
nandrolone
pct
peptide
peptide suggestions
peptides
steroid cycle
steroids
suspension
sustanon
test
test 400
test cyp
test cypionate
test prop
testosterone
testosterone boosters
testosterone cypionate
testsuspension
tren
tren ace
tren ace buy
trenbolone acetate
winstrol
Popular tags
aas
aas testing
anabolic steroids
anabolics online
anabolid steroids
anadrol
anadrol drol tabs inj
anavar
anavar and winnie
body building
body building supplements
bodybuilder
bodybuilding
bodybuilding steroid test
clenbuterol
cycle
deca tren dosage
deca-durobolin
dianabol
dianabol and oxy
dragon pharma
gear
hcg
hgh
motivation
muscle building
muscle mass
nandrolone
pct
peptide
peptide suggestions
peptides
steroid cycle
steroids
suspension
sustanon
test
test 400
test cyp
test cypionate
test prop
testosterone
testosterone boosters
testosterone cypionate
testsuspension
tren
tren ace
tren ace buy
trenbolone acetate
winstrol
Members online
- The OC
- gib68sg
- staatz12
- jray26
- Daswiz
- derzwerg
- lm89
- slenoch81
- hawkmoon
- pompidoux
- AMERICAN SUPPLY
- Eternalpain
- Virtues
- rb1013
- Dave24po1
- Bigdog727
- kitchenchemist
- WeakFNG
- SMOLWARRIOR
- ManeulHuong
- Communion
- biglizard225
- Veneficus_Rex
- UnitedPeptides
- btails
- ironjunkie777
- musclemuscle
- bones77
- chooch
- DBar
- MooseKnuckles
- Dens228
- brokeneck
- Campeon
- goloco
- demon
- gtihonov
- SunrisePharma
- crixus
- buck
- Fa Seeshus
- ryjenn
- bount127
- Canuck54
- Dozerpush1437
- cracker backer
- Prowrestler
- johnjay06
- Nycguy
- Jukaholic
Total: 1,446 (members: 1,438, guests: 8)
Forum statistics
- Total page views
- 636,852,157
- Threads
- 142,358
- Messages
- 2,950,508
- Members
- 182,513
- Latest member
- vic892