Well Dat I was about to ask if adding Test to a GHRH/GHRP anti-aging protocol would be beneficial and now I don't have to bug you with it. But I would like to know if this combination can be continued indefinitely, and whether effective T dosage would be less for anti-aging than bulking. Also whether insulin would be a third useful addition, since prior discussion of insulin has been mostly concerned with adding muscle mass. Actually I'd prefer just the first two, since they're simpler to use, that is low-risk.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Dat's - CJC-1295 & GHRP-6 (Basic Guides)
- Thread starter DatBtrue
- Start date
- Joined
- Jul 25, 2008
- Messages
- 1,700
First of all I am not a doctor...so that means I can't charge you for this consultation.  No...it means I ain't a doctor!
No...it means I ain't a doctor!
Blood work is important because it gives you an objective measure of your current situation. In fact blood work isn't enough...the blood work needs to be proper blood work. A single sampling of GH levels in plasma is no good because they could have sampled during a GH wave or trough.
If you are an older person it is almost a certainty that you produce less GH then you did when you were a youth. Are you GH deficient? As defined by the wider/conventional medical establishment probably not.
By the age of 60 years, however you will be pretty damn GH deficient by most standards.
So in my opinion if you use the peptides described in this thread to restore your levels to that of youth you likely will benefit by increasing the quality of your life.
However to attempt to extend the length of your lifespan you need to hinder the intracellular pathways that IGF-1 promotes. High IGF-1 levels are not good if you want to extend the lifespan beyond normal.
I would love to figure out how to increase the autocrine GH, IGF-1 & MGF in muscle and organs that need it to have a youthful restoration of function while drastically curtailing the intracellular pathways of systemic IGF-1.
Anyway insulin is very much similar to IGF-1 in that the intracellular pathways promote aging...hinder longevity. Elevated insulin is NOT an anti-aging hormone. It can be harmful.
In fact certain steps should be taken to help glucose disposal and reduce the body's need for elevated insulin. There are many ways to attempt this. At the moment I am playing with macadamia oil. *
Testosterone is not something someone should attempt to self-medicate with for HRT or anti-aging...
Assuming you are deficient...and this is a big assumption because there is wide variability...it is very tricky to get the right balance between testosterone and the other hormones that testosterone can convert to. This requires a good HRT specialist such as Dr. Crisler, many bouts of blood work and unfortunately once you start it is very difficult to stop and expect to get your natural levels back to where they were.
Thats why testosterone supplementation should ONLY be used under the care of a doctor and IF you are very deficient. You can start defining deficiency ahead of the blood work by how you feel. If the symptoms are so bad that life sucks and your bloodwork comes back low then you should consider testosterone replacement.
In my opinion for anti-aging you can use the GHRH & GHRP-6 to restore GH to youthful levels and reduce estrogen by eliminating the fat pad (and thus aromatase) with diet & exercise. That will give you a good start.
Blood work is important because it gives you an objective measure of your current situation. In fact blood work isn't enough...the blood work needs to be proper blood work. A single sampling of GH levels in plasma is no good because they could have sampled during a GH wave or trough.
If you are an older person it is almost a certainty that you produce less GH then you did when you were a youth. Are you GH deficient? As defined by the wider/conventional medical establishment probably not.
By the age of 60 years, however you will be pretty damn GH deficient by most standards.
So in my opinion if you use the peptides described in this thread to restore your levels to that of youth you likely will benefit by increasing the quality of your life.
However to attempt to extend the length of your lifespan you need to hinder the intracellular pathways that IGF-1 promotes. High IGF-1 levels are not good if you want to extend the lifespan beyond normal.
I would love to figure out how to increase the autocrine GH, IGF-1 & MGF in muscle and organs that need it to have a youthful restoration of function while drastically curtailing the intracellular pathways of systemic IGF-1.
Anyway insulin is very much similar to IGF-1 in that the intracellular pathways promote aging...hinder longevity. Elevated insulin is NOT an anti-aging hormone. It can be harmful.
In fact certain steps should be taken to help glucose disposal and reduce the body's need for elevated insulin. There are many ways to attempt this. At the moment I am playing with macadamia oil. *
Testosterone is not something someone should attempt to self-medicate with for HRT or anti-aging...
Assuming you are deficient...and this is a big assumption because there is wide variability...it is very tricky to get the right balance between testosterone and the other hormones that testosterone can convert to. This requires a good HRT specialist such as Dr. Crisler, many bouts of blood work and unfortunately once you start it is very difficult to stop and expect to get your natural levels back to where they were.
Thats why testosterone supplementation should ONLY be used under the care of a doctor and IF you are very deficient. You can start defining deficiency ahead of the blood work by how you feel. If the symptoms are so bad that life sucks and your bloodwork comes back low then you should consider testosterone replacement.
In my opinion for anti-aging you can use the GHRH & GHRP-6 to restore GH to youthful levels and reduce estrogen by eliminating the fat pad (and thus aromatase) with diet & exercise. That will give you a good start.
* - In short in animals "fat synthesis" in adipose tissue results in a creation of a "messenger" called Palmitoleate which makes the liver & muscle tissue more insulin sensitive...reduces fatty liver and fat stored in muscle tissue. In general adipose tissue uses Palmitoleate to communicate to distant organs/tissue to, in the words of the study "regulate systemic metabolic homeostasis."
The great unknown is the extent to which this occurs in humans because we don't have as much adipose fat synthesis as animals which is needed to greatly increase Palmitoleate. However macadamia nuts and oil are one of our richest dietary source of this messenger. - Identification of a Lipokine, a Lipid Hormone Linking Adipose Tissue to Systemic Metabolism, Haiming Cao, Cell 134, 933–944, September 19, 2008
The great unknown is the extent to which this occurs in humans because we don't have as much adipose fat synthesis as animals which is needed to greatly increase Palmitoleate. However macadamia nuts and oil are one of our richest dietary source of this messenger. - Identification of a Lipokine, a Lipid Hormone Linking Adipose Tissue to Systemic Metabolism, Haiming Cao, Cell 134, 933–944, September 19, 2008
Well Dat I was about to ask if adding Test to a GHRH/GHRP anti-aging protocol would be beneficial and now I don't have to bug you with it. But I would like to know if this combination can be continued indefinitely, and whether effective T dosage would be less for anti-aging than bulking. Also whether insulin would be a third useful addition, since prior discussion of insulin has been mostly concerned with adding muscle mass. Actually I'd prefer just the first two, since they're simpler to use, that is low-risk.
Has the interference of tamoxifen upon GH-induced IGF-1 production been proven in adult males?The Tamoxifen Citrate/Nolva won't noticably interfere with the IGF-1 that comes about from CJC...especially at that low dose.
- Joined
- Jul 25, 2008
- Messages
- 1,700
Has the interference of tamoxifen upon GH-induced IGF-1 production been proven in adult males?
Not males. This is the state of the science:
From: Tamoxifen Inhibition of Estrogen Receptor-–Negative Mouse Mammary Tumorigenesis, Daniel Medina, Cancer Research 65, 3493-3496, April 15, 2005
"Tamoxifen treatment also attenuates GH release in both rats (40) and humans (41) and decreases insulin-like growth factor-I (IGF-I) concentration in serum of rats (42) and humans (41)."
41 - Inhibitory action on GHRH-induced GH secretion of chronic tamoxifen treatment in breast cancer, De Marinis L, Mancini A, Izzi D, et al, Clin Endocrinol 2000;52:681–5
OBJECTIVE: Previous in vitro and in vivo studies on animal models have demonstrated that tamoxifen (TAM) inhibits GH secretion. Studies in humans are conflicting. The aim of this study was to evaluate the effect of chronic TAM treatment on GH secretory dynamics in the presence of negligible endogenous oestrogens, in postmenopausal women with breast cancer.
PATIENTS: Ten female patients were studied over a 6 - 12-month period after surgical therapy, before medical therapy, and during chronic treatment with TAM (20 mg/day p.o.).
...
DISCUSSION:
In agreement with experimental studies on animals, we have confirmed our preliminary data (De Marinis et al., 1996) demonstrating that chronic TAM treatment is able to reduce GH release induced by GHRH when considering both single time points, peak response and AUC values. Moreover, circulating IGF-1 levels are significantly reduced by TAM treatment. Different studies have investigated the effect of TAM on GH secretion in humans, coming to different conclusions. In postmenopausal women with breast cancer some authors showed no change in basal GH levels (Paterson et al., 1983; Lonning et al., 1992). These data agree with the results of our study where basal GH levels remained unchanged during TAM treatment. On the other hand, in a similar set of patients, other authors observed a trend towards increase an in basal GH levels (Fornander et al., 1993).
The GH response to GHRH has been evaluated in other studies. No change in GHRH-induced GH secretion was found during TAM chronic administration in postmenopausal women with breast cancer (Corsello et al., 1998). Similarly, in healthy premenopausal women short-term TAM treatment (30 mg daily for 2 days) did not significantly modify GHRH-induced GH response (Casanueva et al., 1987).
It is important to consider that the length of TAM treatment in these reports was shorter than in our study (6-12 months): GH levels were measured after 8 weeks (Paterson et al., 1983; Corsello et al., 1998), after 3 months (Fornander et al., 1993), after 1-6 months (Lonning et al., 1992) and after 2 days (Casanueva et al., 1987) of TAM treatment. Moreover, in a series of these patients, old age (mean age 75 years) may explain the low mean response of GH to GHRH before and after TAM treatment (Corsello et al., 1998).
A slight but significant increase of GH levels, but no change in GHRH-induced GH release was observed during chronic TAM treatment (60 days) in a study performed in acromegalic subjects (Cozzi et al., 1997). These results must be considered in relation to the particular subset of patients studied.
The stimulatory role of oestrogen on GH secretory dynamics is well known, as thoroughly reviewed by Giustina & Veldhuis (1998). It has been suggested that the effects of TAM on GH secretion could be ascribed to its anti-oestrogenic action (Frohlander & von Schoultz, 1988; Weissberger & Ho, 1993; Metzger & Kerrigan, 1994). On the other hand, since TAM effects have been observed in postmenopausal patients, in the presence of negligible endogenous oestrogens, this action of TAM may be hypothesized to be exerted at a different level than the OR. Alternative hypotheses are that TAM may interfere with GH dynamics at a central level, blocking GHRH secretion and/or inducing an increase in the somatostatinergic tone, or at the pituitary level.
The hypothesis that the effect of TAM on GH secretion may be ascribed to an increase of somatostatinergic tone is supported by a study in rats (Tannenbaum et al., 1992). TAM, administrated subcutaneously, caused a reduction in the amplitude of spontaneous GH secretory bursts as well as mean plasma GH levels. Of considerable interest is the fact that the inhibitory effect of TAM on the in vivo secretion of GH was diminuished by an anti-somatostatin antiserum ('somatostatinmediated' effect).
On the other hand, supporting the hypothesis that TAM directly inhibits GH pituitary secretion, Malaab et al. (1992) have conducted a study using cultures of immature lamb pituitary cells. They demonstrated that, during acute as well as chronic treatment, clinically relevant concentration of TAM (1-10 mmol/l) have a direct dose-related inhibitory effect on GH release from pituitary somatotrophs. The ability of the cultured cells to respond to GHRH was markedly attenuated.
A peripheral action of TAM on IGF-1 synthesis has to be considered. Numerous studies in recent years have demonstrated the important role that IGF-1 has as a mitogen for breast cancer, as well as for other malignancies (Stracke et al., 1988; Yee et al., 1989; Pollak et al., 1991; Cocconi et al., 1992; Pollak et al., 1992).
TAM modulates IGF-1 action by the stimulation of IGF-BP3 local production and, on the other hand, influences IGF-1 concentrations in the microenvironment of tumour cells. Studies on animals demonstrated that TAM inhibits IGF-1 gene expression in the liver and the lung (Huynh et al., 1993), and influences local production by neighbouring cells, including stromal tissue. Several studies have demonstrated that in humans TAM significantly reduces circulating levels of IGF-1 (Colletti et al., 1989; Pollak et al., 1990; Friedl et al., 1992; Lonning et al., 1992; Fornander et al., 1993; Corsello et al., 1998). TAM could exert this action in peripheral sites, partly due to its weak oestrogenic activity (Murphy & Friesen, 1998). Even if our data do not allow definite conclusions to be drawn on TAM-induced inhibition of spontaneous GH secretion, they suggest that the IGF-1 decrease can be due, at least in part, to reduced GH release after endogenous GHRH secretion. Moreover, considering the negative feedback of IGF-1 on GH secretion (Berelowitz et al., 1981), the inhibitory effect of TAM on GH secretory dynamics despite the reduction of serum IGF- 1 levels further supports the hypothesis of a TAM central action on the GHRH-GH-IGF-1 axis.
A significant inverse correlation between SHBG and IGF-1 circulating levels has been found. Previous studies suggested an influence of IGF-1 on SHBG: first, both proteins are profoundly affected by nutrition in opposite manners; second, it has been suggested that IGF-1 stimulates the process of cellular uptake of SHBG (von Schoultz & Carlstrom, 1989). Our group has previously demonstrated, in a study conducted in pre- and postmenopausal patients with benign breast disease, that lower levels of IGF-1 are present in patients with higher levels of SHBG. This phenomenon occurred only in postmenopausal patients, since IGF-1 is obviously higher and clearly related to GH secretion in premenopausal women (Mancini et al., 1992). However, since it was not present in the group of subjects studied before TAM treatment, partial oestrogenization seems to be necessary to highlight such a relationship.
It is known that TAM increases plasma levels of SHBG in postmenopausal breast cancer patients, probably acting as an oestrogen agonist (Lonning et al., 1995). Yet our results suggest a link with the drug's inhibitory effect on GHRH-GH-IGF-1 axis
In conclusion, our data reinforce the hypothesis of a central action of tamoxifen resulting in inhibition of GH and IGF-1 levels in humans. Keeping in mind the demonstrated mitogenic role of IGF-1 in cancer proliferation, the results of this study may contribute to clarifying the mechanism by which tamoxifen exerts its antiproliferative effect.
PATIENTS: Ten female patients were studied over a 6 - 12-month period after surgical therapy, before medical therapy, and during chronic treatment with TAM (20 mg/day p.o.).
...
DISCUSSION:
In agreement with experimental studies on animals, we have confirmed our preliminary data (De Marinis et al., 1996) demonstrating that chronic TAM treatment is able to reduce GH release induced by GHRH when considering both single time points, peak response and AUC values. Moreover, circulating IGF-1 levels are significantly reduced by TAM treatment. Different studies have investigated the effect of TAM on GH secretion in humans, coming to different conclusions. In postmenopausal women with breast cancer some authors showed no change in basal GH levels (Paterson et al., 1983; Lonning et al., 1992). These data agree with the results of our study where basal GH levels remained unchanged during TAM treatment. On the other hand, in a similar set of patients, other authors observed a trend towards increase an in basal GH levels (Fornander et al., 1993).
The GH response to GHRH has been evaluated in other studies. No change in GHRH-induced GH secretion was found during TAM chronic administration in postmenopausal women with breast cancer (Corsello et al., 1998). Similarly, in healthy premenopausal women short-term TAM treatment (30 mg daily for 2 days) did not significantly modify GHRH-induced GH response (Casanueva et al., 1987).
It is important to consider that the length of TAM treatment in these reports was shorter than in our study (6-12 months): GH levels were measured after 8 weeks (Paterson et al., 1983; Corsello et al., 1998), after 3 months (Fornander et al., 1993), after 1-6 months (Lonning et al., 1992) and after 2 days (Casanueva et al., 1987) of TAM treatment. Moreover, in a series of these patients, old age (mean age 75 years) may explain the low mean response of GH to GHRH before and after TAM treatment (Corsello et al., 1998).
A slight but significant increase of GH levels, but no change in GHRH-induced GH release was observed during chronic TAM treatment (60 days) in a study performed in acromegalic subjects (Cozzi et al., 1997). These results must be considered in relation to the particular subset of patients studied.
The stimulatory role of oestrogen on GH secretory dynamics is well known, as thoroughly reviewed by Giustina & Veldhuis (1998). It has been suggested that the effects of TAM on GH secretion could be ascribed to its anti-oestrogenic action (Frohlander & von Schoultz, 1988; Weissberger & Ho, 1993; Metzger & Kerrigan, 1994). On the other hand, since TAM effects have been observed in postmenopausal patients, in the presence of negligible endogenous oestrogens, this action of TAM may be hypothesized to be exerted at a different level than the OR. Alternative hypotheses are that TAM may interfere with GH dynamics at a central level, blocking GHRH secretion and/or inducing an increase in the somatostatinergic tone, or at the pituitary level.
The hypothesis that the effect of TAM on GH secretion may be ascribed to an increase of somatostatinergic tone is supported by a study in rats (Tannenbaum et al., 1992). TAM, administrated subcutaneously, caused a reduction in the amplitude of spontaneous GH secretory bursts as well as mean plasma GH levels. Of considerable interest is the fact that the inhibitory effect of TAM on the in vivo secretion of GH was diminuished by an anti-somatostatin antiserum ('somatostatinmediated' effect).
On the other hand, supporting the hypothesis that TAM directly inhibits GH pituitary secretion, Malaab et al. (1992) have conducted a study using cultures of immature lamb pituitary cells. They demonstrated that, during acute as well as chronic treatment, clinically relevant concentration of TAM (1-10 mmol/l) have a direct dose-related inhibitory effect on GH release from pituitary somatotrophs. The ability of the cultured cells to respond to GHRH was markedly attenuated.
A peripheral action of TAM on IGF-1 synthesis has to be considered. Numerous studies in recent years have demonstrated the important role that IGF-1 has as a mitogen for breast cancer, as well as for other malignancies (Stracke et al., 1988; Yee et al., 1989; Pollak et al., 1991; Cocconi et al., 1992; Pollak et al., 1992).
TAM modulates IGF-1 action by the stimulation of IGF-BP3 local production and, on the other hand, influences IGF-1 concentrations in the microenvironment of tumour cells. Studies on animals demonstrated that TAM inhibits IGF-1 gene expression in the liver and the lung (Huynh et al., 1993), and influences local production by neighbouring cells, including stromal tissue. Several studies have demonstrated that in humans TAM significantly reduces circulating levels of IGF-1 (Colletti et al., 1989; Pollak et al., 1990; Friedl et al., 1992; Lonning et al., 1992; Fornander et al., 1993; Corsello et al., 1998). TAM could exert this action in peripheral sites, partly due to its weak oestrogenic activity (Murphy & Friesen, 1998). Even if our data do not allow definite conclusions to be drawn on TAM-induced inhibition of spontaneous GH secretion, they suggest that the IGF-1 decrease can be due, at least in part, to reduced GH release after endogenous GHRH secretion. Moreover, considering the negative feedback of IGF-1 on GH secretion (Berelowitz et al., 1981), the inhibitory effect of TAM on GH secretory dynamics despite the reduction of serum IGF- 1 levels further supports the hypothesis of a TAM central action on the GHRH-GH-IGF-1 axis.
A significant inverse correlation between SHBG and IGF-1 circulating levels has been found. Previous studies suggested an influence of IGF-1 on SHBG: first, both proteins are profoundly affected by nutrition in opposite manners; second, it has been suggested that IGF-1 stimulates the process of cellular uptake of SHBG (von Schoultz & Carlstrom, 1989). Our group has previously demonstrated, in a study conducted in pre- and postmenopausal patients with benign breast disease, that lower levels of IGF-1 are present in patients with higher levels of SHBG. This phenomenon occurred only in postmenopausal patients, since IGF-1 is obviously higher and clearly related to GH secretion in premenopausal women (Mancini et al., 1992). However, since it was not present in the group of subjects studied before TAM treatment, partial oestrogenization seems to be necessary to highlight such a relationship.
It is known that TAM increases plasma levels of SHBG in postmenopausal breast cancer patients, probably acting as an oestrogen agonist (Lonning et al., 1995). Yet our results suggest a link with the drug's inhibitory effect on GHRH-GH-IGF-1 axis
In conclusion, our data reinforce the hypothesis of a central action of tamoxifen resulting in inhibition of GH and IGF-1 levels in humans. Keeping in mind the demonstrated mitogenic role of IGF-1 in cancer proliferation, the results of this study may contribute to clarifying the mechanism by which tamoxifen exerts its antiproliferative effect.
Thank you. This is my point: that Nolvadex interferes with IGF-1 production is accepted widely across the Boards, as if it is well-proven fact, but has never been shown to be true for adult males in general, nor adult males supplementing androgens in particular.Not males. This is the state of the science:
From: Tamoxifen Inhibition of Estrogen Receptor-–Negative Mouse Mammary Tumorigenesis, Daniel Medina, Cancer Research 65, 3493-3496, April 15, 2005
"Tamoxifen treatment also attenuates GH release in both rats (40) and humans (41) and decreases insulin-like growth factor-I (IGF-I) concentration in serum of rats (42) and humans (41)."
41 - Inhibitory action on GHRH-induced GH secretion of chronic tamoxifen treatment in breast cancer, De Marinis L, Mancini A, Izzi D, et al, Clin Endocrinol 2000;52:681–5
OBJECTIVE: Previous in vitro and in vivo studies on animal models have demonstrated that tamoxifen (TAM) inhibits GH secretion. Studies in humans are conflicting. The aim of this study was to evaluate the effect of chronic TAM treatment on GH secretory dynamics in the presence of negligible endogenous oestrogens, in postmenopausal women with breast cancer.
PATIENTS: Ten female patients were studied over a 6 - 12-month period after surgical therapy, before medical therapy, and during chronic treatment with TAM (20 mg/day p.o.).
...
DISCUSSION:
In agreement with experimental studies on animals, we have confirmed our preliminary data (De Marinis et al., 1996) demonstrating that chronic TAM treatment is able to reduce GH release induced by GHRH when considering both single time points, peak response and AUC values. Moreover, circulating IGF-1 levels are significantly reduced by TAM treatment. Different studies have investigated the effect of TAM on GH secretion in humans, coming to different conclusions. In postmenopausal women with breast cancer some authors showed no change in basal GH levels (Paterson et al., 1983; Lonning et al., 1992). These data agree with the results of our study where basal GH levels remained unchanged during TAM treatment. On the other hand, in a similar set of patients, other authors observed a trend towards increase an in basal GH levels (Fornander et al., 1993).
The GH response to GHRH has been evaluated in other studies. No change in GHRH-induced GH secretion was found during TAM chronic administration in postmenopausal women with breast cancer (Corsello et al., 1998). Similarly, in healthy premenopausal women short-term TAM treatment (30 mg daily for 2 days) did not significantly modify GHRH-induced GH response (Casanueva et al., 1987).
It is important to consider that the length of TAM treatment in these reports was shorter than in our study (6-12 months): GH levels were measured after 8 weeks (Paterson et al., 1983; Corsello et al., 1998), after 3 months (Fornander et al., 1993), after 1-6 months (Lonning et al., 1992) and after 2 days (Casanueva et al., 1987) of TAM treatment. Moreover, in a series of these patients, old age (mean age 75 years) may explain the low mean response of GH to GHRH before and after TAM treatment (Corsello et al., 1998).
A slight but significant increase of GH levels, but no change in GHRH-induced GH release was observed during chronic TAM treatment (60 days) in a study performed in acromegalic subjects (Cozzi et al., 1997). These results must be considered in relation to the particular subset of patients studied.
The stimulatory role of oestrogen on GH secretory dynamics is well known, as thoroughly reviewed by Giustina & Veldhuis (1998). It has been suggested that the effects of TAM on GH secretion could be ascribed to its anti-oestrogenic action (Frohlander & von Schoultz, 1988; Weissberger & Ho, 1993; Metzger & Kerrigan, 1994). On the other hand, since TAM effects have been observed in postmenopausal patients, in the presence of negligible endogenous oestrogens, this action of TAM may be hypothesized to be exerted at a different level than the OR. Alternative hypotheses are that TAM may interfere with GH dynamics at a central level, blocking GHRH secretion and/or inducing an increase in the somatostatinergic tone, or at the pituitary level.
The hypothesis that the effect of TAM on GH secretion may be ascribed to an increase of somatostatinergic tone is supported by a study in rats (Tannenbaum et al., 1992). TAM, administrated subcutaneously, caused a reduction in the amplitude of spontaneous GH secretory bursts as well as mean plasma GH levels. Of considerable interest is the fact that the inhibitory effect of TAM on the in vivo secretion of GH was diminuished by an anti-somatostatin antiserum ('somatostatinmediated' effect).
On the other hand, supporting the hypothesis that TAM directly inhibits GH pituitary secretion, Malaab et al. (1992) have conducted a study using cultures of immature lamb pituitary cells. They demonstrated that, during acute as well as chronic treatment, clinically relevant concentration of TAM (1-10 mmol/l) have a direct dose-related inhibitory effect on GH release from pituitary somatotrophs. The ability of the cultured cells to respond to GHRH was markedly attenuated.
A peripheral action of TAM on IGF-1 synthesis has to be considered. Numerous studies in recent years have demonstrated the important role that IGF-1 has as a mitogen for breast cancer, as well as for other malignancies (Stracke et al., 1988; Yee et al., 1989; Pollak et al., 1991; Cocconi et al., 1992; Pollak et al., 1992).
TAM modulates IGF-1 action by the stimulation of IGF-BP3 local production and, on the other hand, influences IGF-1 concentrations in the microenvironment of tumour cells. Studies on animals demonstrated that TAM inhibits IGF-1 gene expression in the liver and the lung (Huynh et al., 1993), and influences local production by neighbouring cells, including stromal tissue. Several studies have demonstrated that in humans TAM significantly reduces circulating levels of IGF-1 (Colletti et al., 1989; Pollak et al., 1990; Friedl et al., 1992; Lonning et al., 1992; Fornander et al., 1993; Corsello et al., 1998). TAM could exert this action in peripheral sites, partly due to its weak oestrogenic activity (Murphy & Friesen, 1998). Even if our data do not allow definite conclusions to be drawn on TAM-induced inhibition of spontaneous GH secretion, they suggest that the IGF-1 decrease can be due, at least in part, to reduced GH release after endogenous GHRH secretion. Moreover, considering the negative feedback of IGF-1 on GH secretion (Berelowitz et al., 1981), the inhibitory effect of TAM on GH secretory dynamics despite the reduction of serum IGF- 1 levels further supports the hypothesis of a TAM central action on the GHRH-GH-IGF-1 axis.
A significant inverse correlation between SHBG and IGF-1 circulating levels has been found. Previous studies suggested an influence of IGF-1 on SHBG: first, both proteins are profoundly affected by nutrition in opposite manners; second, it has been suggested that IGF-1 stimulates the process of cellular uptake of SHBG (von Schoultz & Carlstrom, 1989). Our group has previously demonstrated, in a study conducted in pre- and postmenopausal patients with benign breast disease, that lower levels of IGF-1 are present in patients with higher levels of SHBG. This phenomenon occurred only in postmenopausal patients, since IGF-1 is obviously higher and clearly related to GH secretion in premenopausal women (Mancini et al., 1992). However, since it was not present in the group of subjects studied before TAM treatment, partial oestrogenization seems to be necessary to highlight such a relationship.
It is known that TAM increases plasma levels of SHBG in postmenopausal breast cancer patients, probably acting as an oestrogen agonist (Lonning et al., 1995). Yet our results suggest a link with the drug's inhibitory effect on GHRH-GH-IGF-1 axis
In conclusion, our data reinforce the hypothesis of a central action of tamoxifen resulting in inhibition of GH and IGF-1 levels in humans. Keeping in mind the demonstrated mitogenic role of IGF-1 in cancer proliferation, the results of this study may contribute to clarifying the mechanism by which tamoxifen exerts its antiproliferative effect.
IOW, there is no "science". Food for thought.
- Joined
- Jul 25, 2008
- Messages
- 1,700
Yes there is a SIGNIFICANT difference between males & females especially in the area of sex hormones.
I'm just always happy when the research gets from the rat to the mouse (much more related to humans) and very pleased when it gets to humans.
I think if you are gong to circle something it is that dosing protocols that were not defined as chronic (i.e less then 6 months) in females pretty much fail to demonstrate this inhibition.
So it would seem that male bodybuilders who use low dose Tamox in a PCT need not be concerned about inhibition. As you point out they may never need to worry.
The second thing to circle is how the inhibition seems to occur. This appears to be related to somatostatin. GHRP-6 (and all the GHRPs) inhibit somatostatin physiologically so I would conjecture that GHRP-6 would (should inhibition occur) counter Tamox's negative effect.
I'm just always happy when the research gets from the rat to the mouse (much more related to humans) and very pleased when it gets to humans.
I think if you are gong to circle something it is that dosing protocols that were not defined as chronic (i.e less then 6 months) in females pretty much fail to demonstrate this inhibition.
So it would seem that male bodybuilders who use low dose Tamox in a PCT need not be concerned about inhibition. As you point out they may never need to worry.
The second thing to circle is how the inhibition seems to occur. This appears to be related to somatostatin. GHRP-6 (and all the GHRPs) inhibit somatostatin physiologically so I would conjecture that GHRP-6 would (should inhibition occur) counter Tamox's negative effect.
Thank you. This is my point: that Nolvadex interferes with IGF-1 production is accepted widely across the Boards, as if it is well-proven fact, but has never been shown to be true for adult males in general, nor adult males supplementing androgens in particular.
IOW, there is no "science". Food for thought.
You make even more excellent points in this informative post.First of all I am not a doctor...so that means I can't charge you for this consultation.No...it means I ain't a doctor!
Blood work is important because it gives you an objective measure of your current situation. In fact blood work isn't enough...the blood work needs to be proper blood work. A single sampling of GH levels in plasma is no good because they could have sampled during a GH wave or trough.
If you are an older person it is almost a certainty that you produce less GH then you did when you were a youth. Are you GH deficient? As defined by the wider/conventional medical establishment probably not.
By the age of 60 years, however you will be pretty damn GH deficient by most standards.
So in my opinion if you use the peptides described in this thread to restore your levels to that of youth you likely will benefit by increasing the quality of your life.
However to attempt to extend the length of your lifespan you need to hinder the intracellular pathways that IGF-1 promotes. High IGF-1 levels are not good if you want to extend the lifespan beyond normal.
I would love to figure out how to increase the autocrine GH, IGF-1 & MGF in muscle and organs that need it to have a youthful restoration of function while drastically curtailing the intracellular pathways of systemic IGF-1.
Anyway insulin is very much similar to IGF-1 in that the intracellular pathways promote aging...hinder longevity. Elevated insulin is NOT an anti-aging hormone. It can be harmful.
In fact certain steps should be taken to help glucose disposal and reduce the body's need for elevated insulin. There are many ways to attempt this. At the moment I am playing with macadamia oil. *
Testosterone is not something someone should attempt to self-medicate with for HRT or anti-aging...
Assuming you are deficient...and this is a big assumption because there is wide variability...it is very tricky to get the right balance between testosterone and the other hormones that testosterone can convert to. This requires a good HRT specialist such as Dr. Crisler, many bouts of blood work and unfortunately once you start it is very difficult to stop and expect to get your natural levels back to where they were.
Thats why testosterone supplementation should ONLY be used under the care of a doctor and IF you are very deficient. You can start defining deficiency ahead of the blood work by how you feel. If the symptoms are so bad that life sucks and your bloodwork comes back low then you should consider testosterone replacement.
In my opinion for anti-aging you can use the GHRH & GHRP-6 to restore GH to youthful levels and reduce estrogen by eliminating the fat pad (and thus aromatase) with diet & exercise. That will give you a good start.
* - In short in animals "fat synthesis" in adipose tissue results in a creation of a "messenger" called Palmitoleate which makes the liver & muscle tissue more insulin sensitive...reduces fatty liver and fat stored in muscle tissue. In general adipose tissue uses Palmitoleate to communicate to distant organs/tissue to, in the words of the study "regulate systemic metabolic homeostasis."
The great unknown is the extent to which this occurs in humans because we don't have as much adipose fat synthesis as animals which is needed to greatly increase Palmitoleate. However macadamia nuts and oil are one of our richest dietary source of this messenger. - Identification of a Lipokine, a Lipid Hormone Linking Adipose Tissue to Systemic Metabolism, Haiming Cao, Cell 134, 933–944, September 19, 2008
I wish the doctors who ordered the single GH assays for patients who have come into me would learn your first bolded point.
IGF-1 is currently accepted as state of the art. However, IMPO, there is one single assay, produced by a company in Belgium, offered by the lab I use, which is by and far the definitive assessment of GH fnction in aging adults: the 24 hour GH urine assay. Any way you cut it, it says it all.
Doctors who know what they are doing know what GH deficiency is! LOL
I'm sure all can see why now, based upon the excellent information presented in this very thread, an IGF-1 of 250 using GHRH/GHRP is superior to one of 300-350 through frank GH supplementation.
Insulin use by bodybuilders scares me much more than sex hormone supplementation.
For those who are hypogonadal, especially hypogonadal AND diabetic (both Type I and II) TRT is profoundly effective at driving sugar into cells, via several mechanisms, the best of which is facilitated transport.
If anyone is interested, I will post the results from my patients I now have on the protocol Dat recommends here.
Last edited:
Yes there is a SIGNIFICANT difference between males & females especially in the area of sex hormones.
I'm just always happy when the research gets from the rat to the mouse (much more related to humans) and very pleased when it gets to humans.
I think if you are gong to circle something it is that dosing protocols that were not defined as chronic (i.e less then 6 months) in females pretty much fail to demonstrate this inhibition.
So it would seem that male bodybuilders who use low dose Tamox in a PCT need not be concerned about inhibition. As you point out they may never need to worry.
The second thing to circle is how the inhibition seems to occur. This appears to be related to somatostatin. GHRP-6 (and all the GHRPs) inhibit somatostatin physiologically so I would conjecture that GHRP-6 would (should inhibition occur) counter Tamox's negative effect.
I wish this simple point would spread across the Boards to dispel this widely accepted nonsense.
Yes, the hormonal milieu of adult males and females are so different no extrapolated conclusions are warranted.
The mechanism of estrogen's effects upon GH-induced IGF-1 production is via somatostatin?
Last edited:
- Joined
- Jul 25, 2008
- Messages
- 1,700
SWALE said:The mechanism of estrogen's effects upon GH-induced IGF-1 production is via somatostatin?
If not in whole at least in part.
Tamoxifen attenuates pulsatile growth hormone secretion: mediation in part by somatostatin, GS Tannenbaum, Endocrinology, Vol 130, 3395-3401 1992
Tamoxifen, a partial competitive antagonist to the estrogen receptor, is a potent inhibitor of the proliferation of experimental mammary carcinoma in the rat and is widely used clinically in the treatment of breast cancer. Blockade of estrogen receptors present on neoplastic cells represents the classic mechanism of action of tamoxifen, but the drug has a variety of other actions that may contribute to its antiproliferative properties. While it is recognized that estrogens play an important role in modulating pulsatile GH release, the effect of antagonists to sex steroid receptors on GH secretory dynamics has not previously been described.
In the present study we examined the effect of tamoxifen on pulsatile GH secretion in free-moving adult male and female rats. The drug, when administered in a manner previously shown to be associated with antineoplastic activity, caused a marked suppression of the amplitude of spontaneous GH secretory bursts and significantly reduced mean 6-h plasma GH levels in both sexes compared to those in their respective peanut oil-injected controls. Inhibition of spontaneous GH pulses persisted for up to 7 weeks after tamoxifen administration in both sexes. Immunoneutralization of endogenous somatostatin in tamoxifen-treated male rats completely restored both GH pulse amplitude (121.6 +/- 9.5 vs. 62.5 +/- 13.5 ng/ml in tamoxifen- treated rats given normal sheep serum; P less than 0.02) and mean 6-h plasma GH levels (53.3 +/- 6.6 vs. 17.9 +/- 3.6 ng/ml in normal sheep serum-treated rats; P less than 0.01) to levels observed in our peanut oil-injected controls.
These results demonstrate that 1) tamoxifen has potent inhibitory effects on pulsatile GH secretion; and 2) the blunting of GH pulse amplitude by tamoxifen is mediated at least in part by increased release of endogenous somatostatin. These findings motivate further investigation of the clinical significance of tamoxifen-induced suppression of GH secretion in relation to the antineoplastic activity of this commonly used drug.
Tamoxifen, a partial competitive antagonist to the estrogen receptor, is a potent inhibitor of the proliferation of experimental mammary carcinoma in the rat and is widely used clinically in the treatment of breast cancer. Blockade of estrogen receptors present on neoplastic cells represents the classic mechanism of action of tamoxifen, but the drug has a variety of other actions that may contribute to its antiproliferative properties. While it is recognized that estrogens play an important role in modulating pulsatile GH release, the effect of antagonists to sex steroid receptors on GH secretory dynamics has not previously been described.
In the present study we examined the effect of tamoxifen on pulsatile GH secretion in free-moving adult male and female rats. The drug, when administered in a manner previously shown to be associated with antineoplastic activity, caused a marked suppression of the amplitude of spontaneous GH secretory bursts and significantly reduced mean 6-h plasma GH levels in both sexes compared to those in their respective peanut oil-injected controls. Inhibition of spontaneous GH pulses persisted for up to 7 weeks after tamoxifen administration in both sexes. Immunoneutralization of endogenous somatostatin in tamoxifen-treated male rats completely restored both GH pulse amplitude (121.6 +/- 9.5 vs. 62.5 +/- 13.5 ng/ml in tamoxifen- treated rats given normal sheep serum; P less than 0.02) and mean 6-h plasma GH levels (53.3 +/- 6.6 vs. 17.9 +/- 3.6 ng/ml in normal sheep serum-treated rats; P less than 0.01) to levels observed in our peanut oil-injected controls.
These results demonstrate that 1) tamoxifen has potent inhibitory effects on pulsatile GH secretion; and 2) the blunting of GH pulse amplitude by tamoxifen is mediated at least in part by increased release of endogenous somatostatin. These findings motivate further investigation of the clinical significance of tamoxifen-induced suppression of GH secretion in relation to the antineoplastic activity of this commonly used drug.
SEE: Tamoxifen inhibits GH, GH pulse amplitude and IGF-1 -> http://www.professionalmuscle.com/forums/showthread.php?p=506896#post506896
Last edited:
- Joined
- Jul 25, 2008
- Messages
- 1,700
Again people who use Hexarelin need to be careful. Yes there is some conflicting data among studies but I have seen guys dose with the attitude that more is better.
The following study used healthy men and women average age of 68 who responded well to the GH stimulation test. Each individual received subcutaneous injections of hexarelin at a dose of 1.5 ug/kg body weight twice daily for 16 weeks.
The following study used healthy men and women average age of 68 who responded well to the GH stimulation test. Each individual received subcutaneous injections of hexarelin at a dose of 1.5 ug/kg body weight twice daily for 16 weeks.
Does desensitization to hexarelin occur?, A. Rahim, Growth Hormone & IGF Research 1998, 8, 141-143
Summary
Hexarelin, a potent growth hormone (GH)-releasing peptide, is capable of causing profound GH release in normal individuals. If the GH response to hexarelin in humans becomes appreciably attenuated following long-term administration, this would seriously limit the potential therapeutic use of hexarelin and similar agents.
The effect of twice daily subcutaneous injections of hexarelin on GH release was therefore investigated over a period of 16 weeks in 12 healthy elderly individuals. The mean (-+ SEM) areas under the GH curve (AUCGH) at weeks 0 1, 4 and 16 were 19.1 -+ 2.4, 13.1 -+ 2.3, 12.3 + 2.4 and 10.5 -+ 1.8 ug/l/hour, respectively. There was a significant change in AUCGH over the study period (P = 0.0003). Further analysis showed that the decreases in AUCGH at weeks 4 and 16 were significant (P < 0.05 and P < 0.01, respectively) compared with baseline values.
Four weeks after completion of the 16-week study period, hexarelin was again administered. On this occasion, AUCGH increased significantly compared with that at week 16 (from 10.5 _+ 1.8 to 19.4 _+ 3.7 pg/I/hour; P < 0.05) and was not significantly different compared with that at week 0. These results show that attenuation of the GH response after long-term hexarelin therapy is partial and reversible.
Discussion
A similar partial and reversible attenuated GH response to hexarelin has been demonstrated by Klinger et al. 3 who administered hexarelin intranasally, three times a day, to seven prepubertal constitutionally short children for 6-10 months. At the completion of hexarelin therapy, the mean peak GH response to intravenous hexarelin had decreased by about 75% compared with baseline. Three months after completion of hexarelin therapy, the mean peak GH response had increased to 50% of pretreatment levels.
By comparison, Ghigo et al. 4 were unable to demonstrate a change in AUCGH following 8 clays of intranasal administration of hexarelin in seven normal elderly individuals. Differences in dose (which was three times higher in the paediatric study 3 than the study in adults 4) and duration of administration (6-10 months 3 compared with 8 days 4) may account, in part, for the difference between the results obtained by Klinger et al.3 and Ghigo et al., 4 as the route of administration was the same in both studies.
Ghigo et al.4 also found that the response to oral hexarelin in seven elderly women was not attenuated over 15 days of treatment. This finding is in agreement with the results presented here, where there was no significant reduction in the GH response after 1 week of therapy.
Summary
Hexarelin, a potent growth hormone (GH)-releasing peptide, is capable of causing profound GH release in normal individuals. If the GH response to hexarelin in humans becomes appreciably attenuated following long-term administration, this would seriously limit the potential therapeutic use of hexarelin and similar agents.
The effect of twice daily subcutaneous injections of hexarelin on GH release was therefore investigated over a period of 16 weeks in 12 healthy elderly individuals. The mean (-+ SEM) areas under the GH curve (AUCGH) at weeks 0 1, 4 and 16 were 19.1 -+ 2.4, 13.1 -+ 2.3, 12.3 + 2.4 and 10.5 -+ 1.8 ug/l/hour, respectively. There was a significant change in AUCGH over the study period (P = 0.0003). Further analysis showed that the decreases in AUCGH at weeks 4 and 16 were significant (P < 0.05 and P < 0.01, respectively) compared with baseline values.
Four weeks after completion of the 16-week study period, hexarelin was again administered. On this occasion, AUCGH increased significantly compared with that at week 16 (from 10.5 _+ 1.8 to 19.4 _+ 3.7 pg/I/hour; P < 0.05) and was not significantly different compared with that at week 0. These results show that attenuation of the GH response after long-term hexarelin therapy is partial and reversible.
Discussion
A similar partial and reversible attenuated GH response to hexarelin has been demonstrated by Klinger et al. 3 who administered hexarelin intranasally, three times a day, to seven prepubertal constitutionally short children for 6-10 months. At the completion of hexarelin therapy, the mean peak GH response to intravenous hexarelin had decreased by about 75% compared with baseline. Three months after completion of hexarelin therapy, the mean peak GH response had increased to 50% of pretreatment levels.
By comparison, Ghigo et al. 4 were unable to demonstrate a change in AUCGH following 8 clays of intranasal administration of hexarelin in seven normal elderly individuals. Differences in dose (which was three times higher in the paediatric study 3 than the study in adults 4) and duration of administration (6-10 months 3 compared with 8 days 4) may account, in part, for the difference between the results obtained by Klinger et al.3 and Ghigo et al., 4 as the route of administration was the same in both studies.
Ghigo et al.4 also found that the response to oral hexarelin in seven elderly women was not attenuated over 15 days of treatment. This finding is in agreement with the results presented here, where there was no significant reduction in the GH response after 1 week of therapy.
References
3. Klinger B, Sflbergeld A, Deghenghi R, Frenkel J, Laron Z. Desensitization from long-term intranasal treatment with hexarelin does not imerfere with the biological effects of this growth hormone-releasing peptide in short children. Eur J Endocrinol 1996; 134: 716-719.
4. Ghigo E, Arvat E, Gianotti Let al. Short-term administration of intranasal or oral hexarelin, a synthetic hexapeptide, does not desensitize the growth hormone responsiveness in human aging, Eur J Endocrinol 1996; 135: 407-412.
3. Klinger B, Sflbergeld A, Deghenghi R, Frenkel J, Laron Z. Desensitization from long-term intranasal treatment with hexarelin does not imerfere with the biological effects of this growth hormone-releasing peptide in short children. Eur J Endocrinol 1996; 134: 716-719.
4. Ghigo E, Arvat E, Gianotti Let al. Short-term administration of intranasal or oral hexarelin, a synthetic hexapeptide, does not desensitize the growth hormone responsiveness in human aging, Eur J Endocrinol 1996; 135: 407-412.
Thank you! I had not seen that study until now.
Yes, we know E plays an important role in this:
“E2 increased percentages of AP cells with GH protein or mRNA in the aged rats to young levels.”
Mary Iruthayanathan, et al. Department of Neurobiology and Developmental Sciences, College of Medicine, University of Arkansas for Medical Sciences.
The same study supposes DHEA in part and parcel of the process:
“DHEA treatment increased serum GH 1.8 fold…GHRH target cells also increased.”
This is why my humble addition to this protocol is to make sure my "Un-GHRT" patients also take DHEA 25mg BID. I think troches (sublingual lozenges) are best not only because they more effectively elevate serum DHEA, without undue subsequent conversion to estrogen, but also increase DHEA/DHEA-S ratio.
The secondary hypogonadism induced by TRT (even while treating primary hypogonadism) lowers DHEA, which why I "backfill" with same in my TRT patients (along with HCG and PREG TD).
Yes, we know E plays an important role in this:
“E2 increased percentages of AP cells with GH protein or mRNA in the aged rats to young levels.”
Mary Iruthayanathan, et al. Department of Neurobiology and Developmental Sciences, College of Medicine, University of Arkansas for Medical Sciences.
The same study supposes DHEA in part and parcel of the process:
“DHEA treatment increased serum GH 1.8 fold…GHRH target cells also increased.”
This is why my humble addition to this protocol is to make sure my "Un-GHRT" patients also take DHEA 25mg BID. I think troches (sublingual lozenges) are best not only because they more effectively elevate serum DHEA, without undue subsequent conversion to estrogen, but also increase DHEA/DHEA-S ratio.
The secondary hypogonadism induced by TRT (even while treating primary hypogonadism) lowers DHEA, which why I "backfill" with same in my TRT patients (along with HCG and PREG TD).
- Joined
- Jul 25, 2008
- Messages
- 1,700
The DHEA must significantly add to the GH if you are only taking GHRP-6 because a poster on AM first noted it by subjective feel. He was only taking GHRP-6 and added the DHEA and noticed enough of a difference to ask me about it.
Last edited:
- Joined
- Jul 25, 2008
- Messages
- 1,700
Revisiting peptide timing, meals and GH release
This is an interesting study done in cattle. Apparently cattle get one 2 hour feeding a day.
So one hour before the meal GHRH by itself or GHRP-6 by itself worked better then when administered by itself one hour post-meal. That is what we would expect.
In addition one hour pre-meal the GHRH + GHRP-6 produced a larger pulse of GH together. This we would also expect.
What is surprising and interesting is that taking GHRP-6 + GHRH together one hour post-meal produced a pulse of GH basically equivalent to the pre-meal pulse. In other words the synergy of the two peptides over came the meal refractory effect (where either one administered alone was unable).
Perhaps a similar effect takes place in humans... i.e. even when the stomach is full (1 hour post-meal) GHRH+GHRP-6 creates an undiminished GH pulse.
This is an interesting study done in cattle. Apparently cattle get one 2 hour feeding a day.
So one hour before the meal GHRH by itself or GHRP-6 by itself worked better then when administered by itself one hour post-meal. That is what we would expect.
In addition one hour pre-meal the GHRH + GHRP-6 produced a larger pulse of GH together. This we would also expect.
What is surprising and interesting is that taking GHRP-6 + GHRH together one hour post-meal produced a pulse of GH basically equivalent to the pre-meal pulse. In other words the synergy of the two peptides over came the meal refractory effect (where either one administered alone was unable).
Perhaps a similar effect takes place in humans... i.e. even when the stomach is full (1 hour post-meal) GHRH+GHRP-6 creates an undiminished GH pulse.
GH-releasing peptide-6 overcomes refractoriness of somatotropes to GHRH after feeding, C D McMahon, Journal of Endocrinology (2001) 170, 235–241
ABSTRACT
After a meal, somatotropes are temporarily refractory to growth hormone-releasing hormone (GHRH), the principal hormone that stimulates secretion of growth hormone (GH). Refractoriness is particularly evident when free access to feed is restricted to a 2-h period each day. GH-releasing peptide-6 (GHRP-6), a synthetic peptide, also stimulates secretion of GH from somatotropes. Because GHRH and GHRP-6 act via different receptors, we hypothesized that GHRP-6 would increase GHRH-induced secretion of GH after feeding. Initially, we determined that intravenous injection of GHRP-6 at 1, 3 and 10 ug/kg body weight (BW) stimulated secretion of GH in a dose-dependent manner.
Next, we determined that GHRP-6- and GHRH-induced secretion of GH was lower 1 h after feeding (22.5 and 20 ng/ml respectively) than 1 h before feeding (53.5 and 64.5 ng/ml respectively; pooled (S.E.M.=8.5).
However, a combination of GHRP-6 at 3 ug/kg BW and GHRH at 0.2 ug/kg BW synergistically induced an equal and massive release of GH before and after feeding that was fivefold greater than GHRH induced release of GH after feeding.
...
ABSTRACT
After a meal, somatotropes are temporarily refractory to growth hormone-releasing hormone (GHRH), the principal hormone that stimulates secretion of growth hormone (GH). Refractoriness is particularly evident when free access to feed is restricted to a 2-h period each day. GH-releasing peptide-6 (GHRP-6), a synthetic peptide, also stimulates secretion of GH from somatotropes. Because GHRH and GHRP-6 act via different receptors, we hypothesized that GHRP-6 would increase GHRH-induced secretion of GH after feeding. Initially, we determined that intravenous injection of GHRP-6 at 1, 3 and 10 ug/kg body weight (BW) stimulated secretion of GH in a dose-dependent manner.
Next, we determined that GHRP-6- and GHRH-induced secretion of GH was lower 1 h after feeding (22.5 and 20 ng/ml respectively) than 1 h before feeding (53.5 and 64.5 ng/ml respectively; pooled (S.E.M.=8.5).
However, a combination of GHRP-6 at 3 ug/kg BW and GHRH at 0.2 ug/kg BW synergistically induced an equal and massive release of GH before and after feeding that was fivefold greater than GHRH induced release of GH after feeding.
...
- Joined
- Jul 25, 2008
- Messages
- 1,700
Yes, we know E plays an important role in this:
E2 Supplementation Selectively Relieves GH’s Autonegative Feedback on GH-Releasing Peptide-2- Stimulated GH Secretion, Stacey M. Anderson, The Journal of Clinical Endocrinology & Metabolism 86(12):5904–5911 2001
...
In the absence of exogenously imposed GH autofeedback, E2 replacement enhanced the stimulatory effect of GHRP-2 on incremental peak GH release by 1.58-fold [95% confidence interval, 1.2- to 2.1- fold] (P = 0.0034) but did not alter the action of GHRH (0.83- fold [0.62- to 1.1-fold]).
In the E2-deficient state, bolus GH infusion significantly inhibited subsequent spontaneous, GHRH-, and GHRP-induced incremental peak GH responses by, respectively, 33% (1–55%;P = 0.044 vs. saline), 79% (68–86%; P < 0.0001), and 54% (32–69%; P = 0.0002).
E2 repletion failed to influence GH autofeedback on either spontaneous or GHRH-stimulated incremental peak GH output. In contrast, E2 replenishment augmented the GHRP-2-stimulated incremental peak GH response in the face of GH autoinhibition by 1.7-fold (1.2- to 2.5-fold; P = 0.009). Mechanistically, the latter effect of E2 mirrored its enhancement of GH-repressed/GHRP- 2-stimulated GH secretory pulse mass, which rose by 1.5-fold (0.95- to 2.5-fold over placebo; P = 0.078).
In summary, the present clinical investigation documents the ability of short term oral E2 supplementation in postmenopausal women to selectively rescue GHRP-2 (but not spontaneous or GHRH)- stimulated GH secretion from autonegative feedback. The secretagogue specificity of E’s relief of GH autoinhibition suggests that this sex steroid may enhance activity of the hypothalamopituitary GHRP-receptor/effector pathway.
...
In the absence of exogenously imposed GH autofeedback, E2 replacement enhanced the stimulatory effect of GHRP-2 on incremental peak GH release by 1.58-fold [95% confidence interval, 1.2- to 2.1- fold] (P = 0.0034) but did not alter the action of GHRH (0.83- fold [0.62- to 1.1-fold]).
In the E2-deficient state, bolus GH infusion significantly inhibited subsequent spontaneous, GHRH-, and GHRP-induced incremental peak GH responses by, respectively, 33% (1–55%;P = 0.044 vs. saline), 79% (68–86%; P < 0.0001), and 54% (32–69%; P = 0.0002).
E2 repletion failed to influence GH autofeedback on either spontaneous or GHRH-stimulated incremental peak GH output. In contrast, E2 replenishment augmented the GHRP-2-stimulated incremental peak GH response in the face of GH autoinhibition by 1.7-fold (1.2- to 2.5-fold; P = 0.009). Mechanistically, the latter effect of E2 mirrored its enhancement of GH-repressed/GHRP- 2-stimulated GH secretory pulse mass, which rose by 1.5-fold (0.95- to 2.5-fold over placebo; P = 0.078).
In summary, the present clinical investigation documents the ability of short term oral E2 supplementation in postmenopausal women to selectively rescue GHRP-2 (but not spontaneous or GHRH)- stimulated GH secretion from autonegative feedback. The secretagogue specificity of E’s relief of GH autoinhibition suggests that this sex steroid may enhance activity of the hypothalamopituitary GHRP-receptor/effector pathway.
- Joined
- Jul 25, 2008
- Messages
- 1,700
Testosterone blunts IGF-1 inhibition of GH
Rexanator led me to Testosterone Blunts Feedback Inhibition of Growth Hormone Secretion by Experimentally Elevated Insulin-Like Growth Factor-I Concentration, Johannes D. Veldhuis, Stacey M. Anderson, Ali Iranmanesh and Cyril Y. Bowers, The Journal of Clinical Endocrinology & Metabolism Vol. 90, No. 3 1613-1617, 2005, where they found:
The results of this study were confirmed in a recent study published this month:
Rexanator led me to Testosterone Blunts Feedback Inhibition of Growth Hormone Secretion by Experimentally Elevated Insulin-Like Growth Factor-I Concentration, Johannes D. Veldhuis, Stacey M. Anderson, Ali Iranmanesh and Cyril Y. Bowers, The Journal of Clinical Endocrinology & Metabolism Vol. 90, No. 3 1613-1617, 2005, where they found:
"...supplementation of a high dose of Te in middle-aged and older men attenuates IGF-I feedback-dependent inhibition of nadir and peak GH secretion."
The results of this study were confirmed in a recent study published this month:
Testosterone Supplementation in Older Men Restrains Insulin-Like Growth Factor’s Dose-Dependent Feedback Inhibition of Pulsatile Growth Hormone Secretion, Johannes D. Veldhuis, Daniel M. Keenan, Joy N. Bailey, Adenborduin Adeniji, John M. Miles, Remberto Paulo, Mihaela Cosma and Cacia Soares-Welch,The Journal of Clinical Endocrinology & Metabolism Vol. 94, No. 1 246-254, 2009
Background: Pulsatile GH secretion declines in older men. The causal mechanisms are unknown. Candidates include deficient feedforward (stimulation) by endogenous secretagogues and excessive feedback (inhibition) by GH or IGF-I due to age and/or relative hypoandrogenemia.
Hypothesis: Testosterone (T) supplementation in healthy older men will restrain negative feedback by systemic concentrations of IGF-I.
Subjects: Twenty-four healthy men (ages, 50 to 75 yr; body mass index, 24 to 30 kg/m2) participated in the study.
Methods: We performed a prospectively randomized, double-blind, placebo-controlled assessment of the impact of pharmacological T supplementation on GH responses to randomly ordered separate-day injections of recombinant human IGF-I doses of 0, 1.0, 1.5, and 2.0 mg/m2.
Analysis: Deconvolution and approximate entropy analyses of pulsatile, basal, and entropic (pattern-sensitive) modes of GH secretion were conducted.
Results: Recombinant human IGF-I injections 1) elevated mean and peak serum IGF-I concentrations dose-dependently (both P < 0.001); 2) suppressed pulsatile GH secretion (P = 0.003), burst mass (P = 0.025), burst number (P = 0.005), interpulse variability (P = 0.032), and basal GH secretion (P = 0.009); and 3) increased secretory pattern regularity (P = 0.020). T administration did not alter experimentally controlled IGF-I concentrations, but it elevated mean GH concentrations (P = 0.015) and stimulated pulsatile GH secretion (frequency P = 0.037, mass per burst P = 0.038). Compared with placebo, T attenuated exogenous IGF-I’s inhibition of GH secretory-burst mass (P < 0.038) without restoring pulse number, basal secretion, or pattern regularity.
Conclusion: The capability of systemic T to mute IGF-I feedback on pulsatile GH secretion suggests a novel mechanism for augmenting GH production.
Background: Pulsatile GH secretion declines in older men. The causal mechanisms are unknown. Candidates include deficient feedforward (stimulation) by endogenous secretagogues and excessive feedback (inhibition) by GH or IGF-I due to age and/or relative hypoandrogenemia.
Hypothesis: Testosterone (T) supplementation in healthy older men will restrain negative feedback by systemic concentrations of IGF-I.
Subjects: Twenty-four healthy men (ages, 50 to 75 yr; body mass index, 24 to 30 kg/m2) participated in the study.
Methods: We performed a prospectively randomized, double-blind, placebo-controlled assessment of the impact of pharmacological T supplementation on GH responses to randomly ordered separate-day injections of recombinant human IGF-I doses of 0, 1.0, 1.5, and 2.0 mg/m2.
Analysis: Deconvolution and approximate entropy analyses of pulsatile, basal, and entropic (pattern-sensitive) modes of GH secretion were conducted.
Results: Recombinant human IGF-I injections 1) elevated mean and peak serum IGF-I concentrations dose-dependently (both P < 0.001); 2) suppressed pulsatile GH secretion (P = 0.003), burst mass (P = 0.025), burst number (P = 0.005), interpulse variability (P = 0.032), and basal GH secretion (P = 0.009); and 3) increased secretory pattern regularity (P = 0.020). T administration did not alter experimentally controlled IGF-I concentrations, but it elevated mean GH concentrations (P = 0.015) and stimulated pulsatile GH secretion (frequency P = 0.037, mass per burst P = 0.038). Compared with placebo, T attenuated exogenous IGF-I’s inhibition of GH secretory-burst mass (P < 0.038) without restoring pulse number, basal secretion, or pattern regularity.
Conclusion: The capability of systemic T to mute IGF-I feedback on pulsatile GH secretion suggests a novel mechanism for augmenting GH production.
- Joined
- Nov 14, 2007
- Messages
- 113
Please
Also are these DHEA troches available sans prescription? My HRT doc is not quite as enlightened as you Dr. Crisler.
Dat, were you saying that if ghrp-6 is used in conjunction with ghrh then the benefits of DHEA would be much less significant as compared to just the ghrp-6 alone?
If anyone is interested, I will post the results from my patients I now have on the protocol Dat recommends here.
Also are these DHEA troches available sans prescription? My HRT doc is not quite as enlightened as you Dr. Crisler.
Dat, were you saying that if ghrp-6 is used in conjunction with ghrh then the benefits of DHEA would be much less significant as compared to just the ghrp-6 alone?
- Joined
- Jul 25, 2008
- Messages
- 1,700
Also are these DHEA troches available sans prescription? My HRT doc is not quite as enlightened as you Dr. Crisler.
Dat, were you saying that if ghrp-6 is used in conjunction with ghrh then the benefits of DHEA would be much less significant as compared to just the ghrp-6 alone?
NO! Sorry about that. What I meant was Wow!
I recalled a post from someone at AM months ago that was using just GHRP-6 and when he added the DHEA he ended up with the GH side-effect of tingling hands. That prompted him to wonder it DHEA was additive in any way.
So my point is that DHEA's contribution must have been significant to get to a point where you feel it.
Now if you are already depleting the GH stores with each pulse (not likely at GHRH & GHRP-6 saturation doses) then adding other things will be of no benefit.
The focus at that point could be on quickly restoring the GH pituitary stores so that there is something to be released in a follow up pulse.
There are differences among people in how quickly this occurs. Most people need several hours between pulses. So administering GHRP-6+GHRH an hour after you just used it to generate a pulse fails to generate a second pulse. BUT thats not true for everybody.
Some minority of people are able to generate a strong second pulse (i.e. responsive to stimulus) an hour after GHRP-6-GHRH created the first pulse.
I believe the cattle study above concerning meals & refractoriness of somatotropes conjectured that the meal (feeding) refilled the pituitary stores of GH so that one hour post meal GHRH+GHRP-6 succeeded in generating a strong GH pulse.
- Joined
- Jul 25, 2008
- Messages
- 1,700
GH leads to creation of IGF-1 in muscle cells & MGF in muscle cells. That autocrine production and use is really the only thing that matters. In other words systemic IGF-1 can do a lot (but not as well) of what autocrine/paracrine IGF does IF a mutation eliminates autocrine/paracrine action. However IF autocrine/paracrine is functional it will function w/ 100% capacity even if there is no systemic IGF-1 present. It appears that autocrine/paracrine action is THE factor within muscle and probably most organs & tissue as well including the brain.
In fact for bodybuilding I conjecture that low systemic (i.e. endocrine) IGF-1 levels would be fine IF we could increase the expression of IGFs in local tissues (i.e. autocrine = within the cell & also paracrine = neighboring cells such as connective tissue).
Here is a post I made in September in my thread at Anabolicminds (keep in mind IGF-IEa is the muscle form of IGF-1 & MGF is an IGF variant that is only expressed in muscle):
In fact for bodybuilding I conjecture that low systemic (i.e. endocrine) IGF-1 levels would be fine IF we could increase the expression of IGFs in local tissues (i.e. autocrine = within the cell & also paracrine = neighboring cells such as connective tissue).
Here is a post I made in September in my thread at Anabolicminds (keep in mind IGF-IEa is the muscle form of IGF-1 & MGF is an IGF variant that is only expressed in muscle):
Cultured muscle cells as a system for the analysis of IGF-I splicing regulation by factors present in the circulation, Velloso,Cristiana P, The Physiological Society (2004) J Physiol 558P, C5
It has recently been shown that muscle cells grown in 3-D collagen matrixes upregulate IGF-I transcript expression in response to stretching of the matrix (Cheema et al., 2004). In the present work we have studied muscle cells in culture with the aim of determining if either or both of the two splice variants of IGF-I would be upregulated when treated with GH and/or IGF-I, in the absence of mechanical signals C2C12 myoblasts were grown to 50% confluency in medium containing 10% foetal calf serum (FCS). The cells were transferred to medium containing i) 1%FCS only, ii) 100 ng/ml rhGH iii) 100 pg/ml IGF or iv) both. In the untreated cells (i) both isoforms (IGF-IEa and MGF) were present. Treatment with rhGH alone lead to an increase in IGF-IEa and MGF of about 3 fold over control (Table 1). However, treatment with IGF-I abolished expression of both isoforms. When used in combination, the inhibitory effect of IGF-I overode the GH stimulation of IGF-IEa and MGF transcription. We conclude that muscle tissue can upregulate IGF-I isoform expression as a direct result of hormonal stimulation or stretch stimuli. The isoforms of IGF-I seem equally sensitive to GH stimulation in vitro. In vivo, a negative feedback mechanism may modulate the action of GH on IGF-I transcription in muscle tissue in by circulating or local IGF-I expression.

Table 1. IGFIa and MGF transcript levels (x 10-8 ng mRNA / µg RNA) in C2C12 cells following GH and IGF-I treatment.
Data are Means ± S.D. of three separate experiments. * Significant difference (P<0.5) relative to control (unpaired t-test).
It has recently been shown that muscle cells grown in 3-D collagen matrixes upregulate IGF-I transcript expression in response to stretching of the matrix (Cheema et al., 2004). In the present work we have studied muscle cells in culture with the aim of determining if either or both of the two splice variants of IGF-I would be upregulated when treated with GH and/or IGF-I, in the absence of mechanical signals C2C12 myoblasts were grown to 50% confluency in medium containing 10% foetal calf serum (FCS). The cells were transferred to medium containing i) 1%FCS only, ii) 100 ng/ml rhGH iii) 100 pg/ml IGF or iv) both. In the untreated cells (i) both isoforms (IGF-IEa and MGF) were present. Treatment with rhGH alone lead to an increase in IGF-IEa and MGF of about 3 fold over control (Table 1). However, treatment with IGF-I abolished expression of both isoforms. When used in combination, the inhibitory effect of IGF-I overode the GH stimulation of IGF-IEa and MGF transcription. We conclude that muscle tissue can upregulate IGF-I isoform expression as a direct result of hormonal stimulation or stretch stimuli. The isoforms of IGF-I seem equally sensitive to GH stimulation in vitro. In vivo, a negative feedback mechanism may modulate the action of GH on IGF-I transcription in muscle tissue in by circulating or local IGF-I expression.
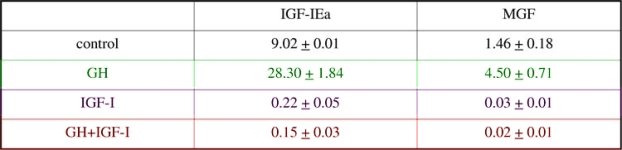
Table 1. IGFIa and MGF transcript levels (x 10-8 ng mRNA / µg RNA) in C2C12 cells following GH and IGF-I treatment.
Data are Means ± S.D. of three separate experiments. * Significant difference (P<0.5) relative to control (unpaired t-test).
- Joined
- Sep 29, 2008
- Messages
- 30
I recalled a post from someone at AM months ago that was using just GHRP-6 and when he added the DHEA he ended up with the GH side-effect of tingling hands. That prompted him to wonder it DHEA was additive in any way.
So my point is that DHEA's contribution must have been significant to get to a point where you feel it.
Do you remember how old that someone was? From the study I understood that DHEA helped restore the GH response to GHRH back to youthful levels in rats, probably through its estrogens metabolites.
Similar threads
Popular tags
aas
aas testing
anabolic steroids
anabolics online
anabolid steroids
anadrol
anadrol drol tabs inj
anavar
anavar and winnie
body building
body building supplements
bodybuilder
bodybuilding
bodybuilding steroid test
clenbuterol
cycle
deca tren dosage
deca-durobolin
dianabol
dianabol and oxy
dragon pharma
gear
hcg
hgh
motivation
muscle building
muscle mass
nandrolone
pct
peptide
peptide suggestions
peptides
steroid cycle
steroids
suspension
sustanon
test
test 400
test cyp
test cypionate
test prop
testosterone
testosterone boosters
testosterone cypionate
testsuspension
tren
tren ace
tren ace buy
trenbolone acetate
winstrol
Popular tags
aas
aas testing
anabolic steroids
anabolics online
anabolid steroids
anadrol
anadrol drol tabs inj
anavar
anavar and winnie
body building
body building supplements
bodybuilder
bodybuilding
bodybuilding steroid test
clenbuterol
cycle
deca tren dosage
deca-durobolin
dianabol
dianabol and oxy
dragon pharma
gear
hcg
hgh
motivation
muscle building
muscle mass
nandrolone
pct
peptide
peptide suggestions
peptides
steroid cycle
steroids
suspension
sustanon
test
test 400
test cyp
test cypionate
test prop
testosterone
testosterone boosters
testosterone cypionate
testsuspension
tren
tren ace
tren ace buy
trenbolone acetate
winstrol
Members online
- Jman88
- rrozell
- jwalk
- IronMindset
- Blobmarine
- shaker89
- td
- Jliltman
- aptry
- Massivekaos
- Eternalpain
- Squat to death
- juiceball44
- HalfwayHulk
- skinnyrunt
- chooch
- Powerlifter
- The OC
- buck
- musclemuscle
- Mike73
- goal245
- LATS
- Berst
- hawkmoon
- scooped
- romo
- Tyrone
- Canuck54
- Elvis755
- mr.e01
- Diesel_Dick
- Haggelus
- uL7iMa
- SuperJew
- dsteelo455
- UnitedPeptides
- ChefTren
- tommyguns2
- KrAven
- qbkilla
- Big Vin
- phani
- Thyak
- mbuilder
- Beachwood
- biglizard225
- brokeneck
- BigGame
- NaDa
Total: 1,450 (members: 1,440, guests: 10)
Forum statistics
- Total page views
- 636,848,940
- Threads
- 142,358
- Messages
- 2,950,490
- Members
- 182,513
- Latest member
- vic892













































































