GHRH & GHRP-2 and GH mRNA & GH-R mRNA
I'm sorry; I should have been more specific. I meant GH mRNA.
The following is the majority of the discussion from, EXPERIMENTAL STUDY - Effect of GHRH and GHRP-2 treatment in vitro on GH secretion and levels of GH, pituitary transcription factor-1, GHRH-receptor, GH-secretagogue-receptor and somatostatin receptor mRNAs in ovine pituitary cells, Ming Yan, Maria Hernandez, Ruwei Xu and Chen Chen, European Journal of Endocrinology (2004) 150 235–242
Its a long read, but I posted it in full because it references relevant in vivo studies as well as their own findings to give us a picture of precisely how GHRH & GHRP-2 effect GH mRNA levels. The timing is interesting...
Note that Sermorelin's half-life comes up a little short....as perhaps 10 minutes is needed.
Solution? - maybe inject Sermorelin & GHRP-6 together, wait 6 minutes and inject another bit of Sermorelin.
Solution? - modify Sermorelin at the 2nd position (swap alanine for D-alanine)
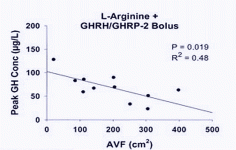
The relevant timing points are highlighted below. As we would expect the combined GHRH (serum made GHRH longer-lasting) + GHRP-2 had the largest impact.
Of interest, GH-receptor mRNA is increased by GHRH immediately as is the GH ligand so it seems that synthesis of GH-receptor triggers early GH release. This point is very interesting to me.
Discussion
...
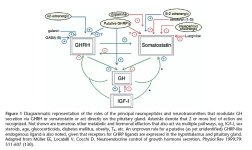
Pituitary GH secretion is, to a large extent, controlled by three regulatory hormones: GHRH, GHS and SRIF. Each binds to G-protein-coupled membrane receptors through which intracellular signalling systems are activated (1). GHRH and GHRP administration potently increases GH secretion and this is not altered by gender, adiposity or age (9). However, peripheral circulating GHRH levels are not usually linked to an increase in GH levels as evidenced in patients with hypothalamic GHRH-secreting tumors (33). The key cell type in the regulation of GH levels is the pituitary somatotrope, which determines the amount of GH secreted in response to hypothalamic GHRH stimulation. Combined GHRH and GHRP treatment plays an important role clinically, however, the data on the mechanism of GHRH/GHRH-R and GHRP/GHS-R action are controversial (19–21). The differences may relate to the duration of GHRH and GHRP treatment and cell culture conditions as well as animal age.
In this study, we investigated the mechanism of action of GHRH/GHRP using primary cultures of ovine pituitary cells treated with GHRH and GHRP-2 in vitro. The present study carefully investigated the concentration and duration of GHRH and GHRP treatment using 0.5, 1, 1.5 and 2 h time points and serum-free incubation conditions for cell culture to clarify the mechanism of GHRH/GHRH-R and GHRP/GHS-R action. Treatments with 10nM GHRH and 100nM GHRP-2 for 0.5, 1, 1.5 and 2 h were chosen in this study to investigate short- to mid-term changes in somatotropes.
GHRH at 1.0 mg/kg (i.v.) in vivo maximally stimulated GH secretion at 15–45 min, with GH levels returning to baseline by 90–120 min after GHRH injection in humans (34). GHRP-2, GHRP-6, hexarelin or non-peptidyl GHRP mimetic compound (L-692, 429) treatment rapidly increases serum GH concentrations within 5–15 min, with the peak GH concentration usually observed 15–30 min after intravenous injection in humans (35, 36). The presence of serum in the culture medium maintains basal levels of the GHRH receptor and is important in long-term GHRH treatment (20).
However, the biological half-life of GHRH 1–44 is about 3–6 min in vivo (37) as GHRH is rapidly inactivated by a plasma dipeptidyl aminopeptidase, producing a more stable metabolite, GHRH 3–44, which is about 1000 times less potent than the parent compound (37). The culture of ovine pituitary cells in serum-free culture conditions, as employed in this study, overcomes the quick degradation of GHRH and allows subsequent stimulatory GHRH and GHRP treatments to be performed under defined conditions (
38).
GHRH and GHRP treatment for 15 and 30 min rapidly stimulates maximal GH release (34–36).
Furthermore, GHRH treatment for as little as 10 min rapidly increased GH transcription rate by 200–300% in primary cultured pituitary cells (39, 40). To analyse the transcription regulation of GH, we examined GH mRNA levels in response to GHRH and GHRP-2. Our results show that treatment with GHRH, GHRP-2 and combinations of GHRH and GHRP-2 increased GH mRNA expression and GH release 0.5, 1.0, 1.5 and 2 h after treatment, in a time-dependent manner. The level of GH mRNA 0.5, 1, 1.5 and 2 h after treatment was greatest in the combined GHRH and GHRP- 2 treatment group rather than in the GHRH or GHRP-2 alone treatment groups. Our results are consistent with early reports that 10nM GHRH treatment of rat pituitary cells in serum-free medium increased GH mRNA expression by 1.8- and almost 2.0-fold at 0.5 and 1 h respectively (40). This demonstrates that the effects of GHRH, GHRP-2 and combinations of GHRH and GHRP-2 on GH mRNA expression are rapid and occur at GH gene transcription level (40).
GHRH and GHRP bind to their specific receptors on the membranes of somatotropes (
37). GHRH stimulates GH synthesis by increasing the transcription rate of the GH gene and consequently GH release (40, 41). GHRP-2 enhances pituitary GH gene expression and directly stimulates GH release (42).
Our results show that the duration of GHRH or GHRP-2 treatment influences the effects on the corresponding receptor mRNA expression and GH release. GHRH and GHRP treatment for 1.5 h reduces their own receptor mRNA levels. These results are consistent with previous reports which suggest that GHRH in the short-term suppresses its own receptor expression (
8, 20).
Surprisingly, in this experiment, the short-term GHRH or GHRP treatment (0.5 h) significantly increased the expression of ligand-specific receptor mRNAs, and also increased GH release from ovine pituitary cells. This suggests that GHRH-R or GHS-R mRNA expression may contribute to the increase in GH secretion. GHRH treatment does not significantly influence the mRNA level of GHS-R, nor does GHRP-2 change GHRH-R expression with short-term treatment. The results supported our previous report suggesting that GHRP-2 does not act through the GHRH receptor (6). It is worth mentioning that another study indicated that GHRH, at any dose tested, did not affect GHS-R levels in vitro (8).
GH expression is mainly controlled by Pit-1, a member of the homobox POU (representing a homeodomain protein family of which the founder members are Pit-1, Oct 1/2 and Unc-86) family of DNA-binding proteins, and GHRH and GHRP-2 elicit a time-dependent activation of Pit-1 expression by anterior pituitary cells (1, 35). Our results indicate that with combined GHRH and GHRP-2 treatment, Pit-1 mRNA expression is increased to 150, 121 and 168% at 0.5, 1.5 and 2 h after treatment respectively. GHRH enhances the levels of Pit-1 mRNA expression 0.5, 1, 1.5 and 2 h after treatment. GHRP-2 also significantly increases the levels of Pit-1 mRNA 0.5 and 2 h after treatment. GHRH and GHRP-2 may activate Pit-1 transcription and stimulates GH expression in pituitary cells through mediation of protein kinase C (PKC), mitogen-activated protein (MAP) kinase and PKA activation (1, 13, 14, 43).
Somatostatin binds to a family of specific receptors and inhibits adenylyl cyclase via Gi proteins, and inhibits GH release but not its biosynthesis (44). In addition, somatostatin may potentially play (dual) inhibitory and stimulatory roles in controlling GH secretion by acting on two distinct somatotrope cell populations in the porcine pituitary (45). Five somatostatin receptor subtypes have been cloned and characterized and their expression is regulated in a subtype and tissue-specific manner (46–48). The results of GHRH action on sst receptor synthesis shows that, in vivo, a 4 h GHRH infusion and, in vitro, a 4 h 10nM GHRH treatment of rat pituitary cells increased sst-1 and sst-2 mRNA levels but decreased sst-5 mRNA levels (26). In the current study, 10 nM GHRH treatment increased sst-1 mRNA expression 0.5 to 2 h after the treatment. Although GHRH also increased sst-2 mRNA expression this was not statistically significant. This suggests that the acute direct regulatory action of GHRH on the synthesis of sst-1 and sst-2 receptor subtypes may be time-dependent. GHRH increased sst-1 mRNA expression may be partially due to GHRH-induced increases in Pit-1 mRNA, which activates pituitary sst-1 mRNA expression (14, 49). In contrast, 100nM GHRP-2 reduced sst-1 and sst-2 mRNA expression 0.5 to 2 h after treatment. GHRP has been suggested to act as a functional SRIF antagonist (11). Inhibition of the SRIF receptors including sst-1 and sst-2 by GHRP-2 supports this view.
In summary, the results of this study indicate that GHRH and GHRP-2 are important mediators regulating GH, GHRH-R, GHS-R, Pit-1, sst-1 and sst-2 mRNA expression, and GH synthesis. Effects on somatotropes manifest as either a priming or an inhibitory modification of the cells, leading to increased or decreased GH secretion. Moreover, the results demonstrate that GHRH and GHRP regulate their receptor synthesis and GH release in a time-dependent manner. This study represents an essential step forward in understanding the influence of GHRH and GHRP on somatotropes. Application of this understanding may aid the development of new GHSs with high efficacy.
Selected References
37 Frohman LA & Kineman RD. Growth hormone-releasing hormone and pituitary somatotrope proliferation. Minerva Endocrinology 2002 27 277–285.
38 Gick GG, Zeytin FN, Brazeau P, Ling NC, Esch FS & Bancroft C. Growth hormone-releasing factor regulates growth hormone mRNA in primary cultures of rat pituitary cells. PNAS 1984 81 1553–1555.
39 Barinaga M, Yamonoto G, Rivier C, Vale W, Evans R & Rosenfeld MG. Transcription regulation of growth hormone gene expression by growth hormone-releasing factor. Nature 1983 306 84–85.
40 Barinaga M, Bilezikjian LM, Vale WW, Rosenfeld MG & Evans RM. Independent effects of growth hormone releasing factor on growth hormone release and gene transcription. Nature 1985 314 279–281.
8 Kineman RD, Kamegai J & Frohman LA. Growth hormone (GH)- releasing hormone (GHRH) and the GH secretagogue (GHS), L692,585, differentially modulate rat pituitary GHS receptor and GHRH receptor messenger ribonucleic acid levels. Endocrinology 1999 140 3581–3586.
20 Lasko CM, Korytko AI, Wehrenberg WB & Cuttler L. Differential GH-releasing hormone regulation of GHRH receptor mRNA expression in the rat pituitary. American Journal of Physiology 2001 280 E626–631.
**broken link removed**