You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Dat's - CJC-1295 & GHRP-6 (Basic Guides)
- Thread starter DatBtrue
- Start date
- Joined
- Nov 29, 2007
- Messages
- 67
I have been on an every other day diet for a couple of months now w/ one of those days below 50% of my maintenance and I feel pretty darn good. The most noticeable effect is a sharper mind/memory although part of that effect comes from my EOD intake of Mucuna pruriens, 40% L-Dopa w/ GHRPs. - **
and as far as building muscle?
- Joined
- Jul 25, 2008
- Messages
- 1,700
and as far as building muscle?
That was not the goal. But I gained a little.
But if you want to build muscle instead of burn fat you would eat more on training day. Add things like glycerol w/ BCAAs and administer insulin.
As I went along I began to "feel" more anabolic on training day as a result of the previous diet day. So it was like a mini "prime" (ala Dorian Yates) and then a mini anabolic rebound. Obviously on a tiny scale.
- Joined
- Nov 27, 2006
- Messages
- 1,481
DatBtrue,
If someone was to utilize GRF(1-29)/GHRP-6 as part of contest preparation, how many days out would it be best to stop the protocol to eliminate fluid retention?
Thank you.
If someone was to utilize GRF(1-29)/GHRP-6 as part of contest preparation, how many days out would it be best to stop the protocol to eliminate fluid retention?
Thank you.
- Joined
- Jan 21, 2009
- Messages
- 648
I wanted to pass this very descriptive video on which discusses DAC Technology. If you are still not technically clear on the difference between CJC 1295 with DAC and 1295 without DAC, this video from Conjuchem should help.
**broken link removed**
**broken link removed**
- Joined
- Jul 25, 2008
- Messages
- 1,700
DatBtrue,
If someone was to utilize GRF(1-29)/GHRP-6 as part of contest preparation, how many days out would it be best to stop the protocol to eliminate fluid retention?
Thank you.
Water retention is an individual thing. I noticed I held a LOT more water with CJC-1295 then I do with mod GRF(1-29). Without diuretics or any other compounds the water was gone in about a weeks time.
- Joined
- Jul 25, 2008
- Messages
- 1,700
I wanted to pass this very descriptive video on which discusses DAC Technology. If you are still not technically clear on the difference between CJC 1295 with DAC and 1295 without DAC, this video from Conjuchem should help.
**broken link removed**
Conjuchem a Canadian firm has the patent on the DAC. As you can see from their website CJC-1295 is not even discussed. They use the DAC for a couple of unrelated products that are (I believe) commercially available or close to it. Plus they license the DAC technology as well.
Nice little video BT.
Been using GHRP/GHRH 100mcgs 3x/day and getting great results. I have nerve damage to my left arm and shoulder which makes my left arm 1 inch smaller than my right. Been thinking of using IGF in my left arm to help out.
DAT - Do you think this would help and can I use it along with the GHRP/GHRH? How much time would I need between the IGF and the GHRP/GHRH?
Any help would be appreciated
DAT - Do you think this would help and can I use it along with the GHRP/GHRH? How much time would I need between the IGF and the GHRP/GHRH?
Any help would be appreciated
- Joined
- Jul 25, 2008
- Messages
- 1,700
IGF-1 a potent neurotrophic factor for motor neurons
The answer to your question is that no one knows for sure. Administering IGF-1 LR3 is different then inducing local expression of IGF-1.
It is the expression of local IGF-1 that seems to have the best results yet IGF-1 infusion in mice has demonstrated positive results. The following taken from a study gives a nice recitation of the current knowledge in the area of IGF-1 & nerve injuries. Spend some time with it...
...I think it is an easy to understand & informative read for all of us.
Targeted Expression of IGF-1 Transgene to Skeletal Muscle Accelerates Muscle and Motor Neuron Regeneration, Eric D. Rabinovsky, The FASEB Journal Express Article doi:10.1096/fj.02-0183fje
From the INTRO:
The repair of peripheral nerves damaged by injury or disease is often incomplete despite vast improvements in microsurgical techniques. There still exists an urgent need to develop new medical treatments that hastens the repair of damaged nerve and muscle. Neurotrophic factors that play a critical role in the development and regeneration of muscle and nerve have the potential for being used as gene therapies to enhance the repair of injured and diseased nerves and muscle. One candidate factor exerting both myogenic and neurotrophic factor activities is insulin-like growth factor-1 (IGF-1).
IGF-1 is a potent neurotrophic factor for motor neurons. It is a survival factor for cultured spinal cord motoneurons and helps to maintain high levels of choline acetyltransferase activity (1, 2). Neurite outgrowth is induced in cultured spinal cord motor neurons both in the presence and absence of non-neuronal cells (3, 4). In vivo studies show that IGF-1 can rescue motoneurons from axotomy-induced death or programmed cell death (2, 5). Local infusion of IGF-1 stimulates nerve regeneration in crushed (6, 7) or freeze-injured sciatic nerve (8). Sensory regeneration in sciatic nerve is enhanced in diabetic rats by exogenous application of IGF-1 near the nerve injury (9). Conversely, local infusion of IGF antiserum significantly inhibits spontaneous regeneration of motor and sensory nerves (10, 11).
Nerve sprouting within skeletal muscles is an essential restorative process in response to an injury or a pathological condition. IGF-1 increases intramuscular nerve sprouting 10-fold when administered subcutaneously to normal adult rats (3). Injection of IGF binding protein 4, an inhibitor of IGF-1 activity, completely blocks the neurite promoting activity of muscle extract (12), further implicating IGF-1 as a neurite sprouting factor. These studies are supported by experiments showing that subcutaneously injected IGF-1 accumulates at the neuromuscular junction and in neuromuscular nerves and induces the expression of the nerve growth associated protein GAP-43 in the motor neuron perikarya (13, 14).
IGF-1 is also a potent myogenic factor, promoting myoblast proliferation, myogenic differentiation, and myotube hypertrophy (15). Evidence of the myogenic effects of IGF-1 are demonstrated by studies showing increased muscle myofiber hypertrophy in transgenic mice overexpressing IGF-1 in skeletal muscle (16). In regenerating muscle IGF-1 expression is increased early in muscle satellite cells and continues to increase through the formation of myofibers (17, 18). These studies suggest that the potential of IGF-1 for inducing muscle repair may be in its ability to function as both a myogenic factor as well as a neurotrophic factor.
Given the effects of IGF-1 in the regeneration of nerve and muscle, we reasoned that localized secretion of IGF-1 in regenerating muscle cells should facilitate motor nerve and muscle repair after nerve injury. This hypothesis was tested in an IGF-1 transgenic line that specifically expresses human IGF-1 in skeletal muscle, via the skeletal a-actin promoter (16). Our studies show that when the sciatic nerve is injured, increased local expression of IGF-1 in muscle hastens motor nerve and muscle repair.
From the CONCLUSION:
In conclusion, these experiments demonstrate that muscle and nerve repair are accelerated by the localized expression of IGF-1 in skeletal muscle. Exerting a myogenic effect, the IGF-1-transgene enhances the activation and differentiation of muscle satellite cells in response to nerve injury. Exerting a neurotrophic effect, the IGF-1 transgene hastens the reestablishment of synaptic connections, as shown by increased innervation and restoration of myogenin and AchR mRNA expression levels and increased nerve conduction rates. The duel role of IGF-1 on motor neuron and muscle regeneration illustrates the benefits of targeting IGF-1 gene expression to skeletal muscle as a potential gene therapeutic application for injured or diseased nerves.
Been using GHRP/GHRH 100mcgs 3x/day and getting great results. I have nerve damage to my left arm and shoulder which makes my left arm 1 inch smaller than my right. Been thinking of using IGF in my left arm to help out.
DAT - Do you think this would help and can I use it along with the GHRP/GHRH? How much time would I need between the IGF and the GHRP/GHRH?
Any help would be appreciated
The answer to your question is that no one knows for sure. Administering IGF-1 LR3 is different then inducing local expression of IGF-1.
It is the expression of local IGF-1 that seems to have the best results yet IGF-1 infusion in mice has demonstrated positive results. The following taken from a study gives a nice recitation of the current knowledge in the area of IGF-1 & nerve injuries. Spend some time with it...
...I think it is an easy to understand & informative read for all of us.
Targeted Expression of IGF-1 Transgene to Skeletal Muscle Accelerates Muscle and Motor Neuron Regeneration, Eric D. Rabinovsky, The FASEB Journal Express Article doi:10.1096/fj.02-0183fje
From the INTRO:
The repair of peripheral nerves damaged by injury or disease is often incomplete despite vast improvements in microsurgical techniques. There still exists an urgent need to develop new medical treatments that hastens the repair of damaged nerve and muscle. Neurotrophic factors that play a critical role in the development and regeneration of muscle and nerve have the potential for being used as gene therapies to enhance the repair of injured and diseased nerves and muscle. One candidate factor exerting both myogenic and neurotrophic factor activities is insulin-like growth factor-1 (IGF-1).
IGF-1 is a potent neurotrophic factor for motor neurons. It is a survival factor for cultured spinal cord motoneurons and helps to maintain high levels of choline acetyltransferase activity (1, 2). Neurite outgrowth is induced in cultured spinal cord motor neurons both in the presence and absence of non-neuronal cells (3, 4). In vivo studies show that IGF-1 can rescue motoneurons from axotomy-induced death or programmed cell death (2, 5). Local infusion of IGF-1 stimulates nerve regeneration in crushed (6, 7) or freeze-injured sciatic nerve (8). Sensory regeneration in sciatic nerve is enhanced in diabetic rats by exogenous application of IGF-1 near the nerve injury (9). Conversely, local infusion of IGF antiserum significantly inhibits spontaneous regeneration of motor and sensory nerves (10, 11).
Nerve sprouting within skeletal muscles is an essential restorative process in response to an injury or a pathological condition. IGF-1 increases intramuscular nerve sprouting 10-fold when administered subcutaneously to normal adult rats (3). Injection of IGF binding protein 4, an inhibitor of IGF-1 activity, completely blocks the neurite promoting activity of muscle extract (12), further implicating IGF-1 as a neurite sprouting factor. These studies are supported by experiments showing that subcutaneously injected IGF-1 accumulates at the neuromuscular junction and in neuromuscular nerves and induces the expression of the nerve growth associated protein GAP-43 in the motor neuron perikarya (13, 14).
IGF-1 is also a potent myogenic factor, promoting myoblast proliferation, myogenic differentiation, and myotube hypertrophy (15). Evidence of the myogenic effects of IGF-1 are demonstrated by studies showing increased muscle myofiber hypertrophy in transgenic mice overexpressing IGF-1 in skeletal muscle (16). In regenerating muscle IGF-1 expression is increased early in muscle satellite cells and continues to increase through the formation of myofibers (17, 18). These studies suggest that the potential of IGF-1 for inducing muscle repair may be in its ability to function as both a myogenic factor as well as a neurotrophic factor.
Given the effects of IGF-1 in the regeneration of nerve and muscle, we reasoned that localized secretion of IGF-1 in regenerating muscle cells should facilitate motor nerve and muscle repair after nerve injury. This hypothesis was tested in an IGF-1 transgenic line that specifically expresses human IGF-1 in skeletal muscle, via the skeletal a-actin promoter (16). Our studies show that when the sciatic nerve is injured, increased local expression of IGF-1 in muscle hastens motor nerve and muscle repair.
From the CONCLUSION:
In conclusion, these experiments demonstrate that muscle and nerve repair are accelerated by the localized expression of IGF-1 in skeletal muscle. Exerting a myogenic effect, the IGF-1-transgene enhances the activation and differentiation of muscle satellite cells in response to nerve injury. Exerting a neurotrophic effect, the IGF-1 transgene hastens the reestablishment of synaptic connections, as shown by increased innervation and restoration of myogenin and AchR mRNA expression levels and increased nerve conduction rates. The duel role of IGF-1 on motor neuron and muscle regeneration illustrates the benefits of targeting IGF-1 gene expression to skeletal muscle as a potential gene therapeutic application for injured or diseased nerves.
REFERENCES:
1. Arakawa, Y., Sendtner, M., Thoenen, H. (1990) Survival effect of ciliary neurotrophic factor (CNTF) on embryonic motoneurons in culture: Comparison with other neurotrophic factors and cytokines. J. Neurosci. 10, 3507?3515
2. Neff, N.T., Prevette, D., Houenou, L., Lewis, M.D., Glicksman, M.A., Yin, Q.W., Oppenheim R.W. (1993) Insulin-like growth factors: Putative muscle-derived trophic agents that promotes motoneuron survival. J. Neurobiol. 24, 1578?1588
3. Caroni, P., Grandes, P. (1990) Nerve sprouting in innervated skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J. Cell Biol. 110, 1307?1317
4. Recio-Pinto, E., Rechler, M.M., Ishii, D.N. (1986) Effects of insulin, insulin-like growth factor-II and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J. Neurosci. 6, 1211?1219
5. Li, L., Oppenheim, R.W., Lei, M., Houenou. L.J. (1994) Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J. Neurobiol. 25, 759?766
6. Kanje, M., Skottner, A., Sjoberg, S.A., Lundborg, G. (1989) Insulin-like growth actor I (IGF-I) stimulates regeneration of the rat sciatic nerve. Brain Res. 486, 396?398
7. Skottner, A., Kanje, M., Widegram, D., Lundborg, G., Logberg, E. (1987) Insulin-like growth factor-I (IGF-I) stimulates peripheral nerve regeneration in rats. J. Clin. Invest. 10, 25
8. Sjoberg, J., Kanje, M. (1989) Insulin-like growth factor I (IGF-I) as a stimulator of regeneration in the freeze-injured rat sciatic nerve. Brain Res. 485, 102?108
9. Ishii, D.N., Lupien, S.B. (1995) Insulin-like growth factors protect against diabetic neuropathy: Effects on sensory nerve regeneration in rats. J. Neurosci. Res. 40, 138?144
10. Near, S.L., Whalen, L.R., Miller, J.A., Ishii. D.N. (1992) Insulin-like growth factor I stimulates motor nerve regeneration. Proc. Natl. Acad. Sci. USA 89, 11716?11720
11. Glazner, G.W., Lupien S., Miller, J.A., Ishii. D.N. (1993) Insulin-like growth factor II increases the rate of sciatic nerve regeneration in rats. Neurosci. 54, 791?797
12. Caroni, P., Schneider, C., Kiefer, M.C., Zapf, J. (1994) Role of muscle insulin-like growth factors in nerve sprouting: suppression of terminal sprouting in paralyzed muscle by IGF-binding protein 4. J. Cell Biol. 125, 893?902
13. Lewis, M.E., Vaught, J.L., Neff, N.T., Grebow, P.E., Callison, K.V., Yu, E., Contreras, P.C., Baldino, F. (1993) The potential of insulin-like growth factor-I as a therapeutic for the treatment of neuromuscular disorders. Ann. N.Y. Acad. Sci. 293, 201?208
14. Caroni, P., Becker. M. (1992) The downregulation of growth-associated proteins in motoneurons at the onset of synapse elimination is controlled by muscle activity and IGF-I. J. Neurosci. 12, 3849?3861
15. Florini, J.R., Ewton, D.Z., Coolican, S.A. (1996) Growth hormone and the insulin-like growth factor system in myogenesis. Endocrine Rev. 17, 481?517
16. Coleman, M.E., DeMayo, F., Yin, K.C., Lee, H.M., Geske, R., Montgomery, C., Schwartz. R.J. (1995) Myogenic vector expression of insulin-like growth factor 1 stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J. Biol. Chem. 270, 12109?12116
17. Jennische, E., Skottner, A., Hansson, H.A. (1987) Satellite cells express the trophic factor IGF-1 in regenerating skeletal muscle cell. Acta Physiol. Scand. 129, 9?15
18. Jannische, E., Hansson. H.A. (1987) Regenerating skeletal muscle cells express insulin-like growth factor I. Acta Physiol. Scand. 130, 327?332
1. Arakawa, Y., Sendtner, M., Thoenen, H. (1990) Survival effect of ciliary neurotrophic factor (CNTF) on embryonic motoneurons in culture: Comparison with other neurotrophic factors and cytokines. J. Neurosci. 10, 3507?3515
2. Neff, N.T., Prevette, D., Houenou, L., Lewis, M.D., Glicksman, M.A., Yin, Q.W., Oppenheim R.W. (1993) Insulin-like growth factors: Putative muscle-derived trophic agents that promotes motoneuron survival. J. Neurobiol. 24, 1578?1588
3. Caroni, P., Grandes, P. (1990) Nerve sprouting in innervated skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J. Cell Biol. 110, 1307?1317
4. Recio-Pinto, E., Rechler, M.M., Ishii, D.N. (1986) Effects of insulin, insulin-like growth factor-II and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J. Neurosci. 6, 1211?1219
5. Li, L., Oppenheim, R.W., Lei, M., Houenou. L.J. (1994) Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J. Neurobiol. 25, 759?766
6. Kanje, M., Skottner, A., Sjoberg, S.A., Lundborg, G. (1989) Insulin-like growth actor I (IGF-I) stimulates regeneration of the rat sciatic nerve. Brain Res. 486, 396?398
7. Skottner, A., Kanje, M., Widegram, D., Lundborg, G., Logberg, E. (1987) Insulin-like growth factor-I (IGF-I) stimulates peripheral nerve regeneration in rats. J. Clin. Invest. 10, 25
8. Sjoberg, J., Kanje, M. (1989) Insulin-like growth factor I (IGF-I) as a stimulator of regeneration in the freeze-injured rat sciatic nerve. Brain Res. 485, 102?108
9. Ishii, D.N., Lupien, S.B. (1995) Insulin-like growth factors protect against diabetic neuropathy: Effects on sensory nerve regeneration in rats. J. Neurosci. Res. 40, 138?144
10. Near, S.L., Whalen, L.R., Miller, J.A., Ishii. D.N. (1992) Insulin-like growth factor I stimulates motor nerve regeneration. Proc. Natl. Acad. Sci. USA 89, 11716?11720
11. Glazner, G.W., Lupien S., Miller, J.A., Ishii. D.N. (1993) Insulin-like growth factor II increases the rate of sciatic nerve regeneration in rats. Neurosci. 54, 791?797
12. Caroni, P., Schneider, C., Kiefer, M.C., Zapf, J. (1994) Role of muscle insulin-like growth factors in nerve sprouting: suppression of terminal sprouting in paralyzed muscle by IGF-binding protein 4. J. Cell Biol. 125, 893?902
13. Lewis, M.E., Vaught, J.L., Neff, N.T., Grebow, P.E., Callison, K.V., Yu, E., Contreras, P.C., Baldino, F. (1993) The potential of insulin-like growth factor-I as a therapeutic for the treatment of neuromuscular disorders. Ann. N.Y. Acad. Sci. 293, 201?208
14. Caroni, P., Becker. M. (1992) The downregulation of growth-associated proteins in motoneurons at the onset of synapse elimination is controlled by muscle activity and IGF-I. J. Neurosci. 12, 3849?3861
15. Florini, J.R., Ewton, D.Z., Coolican, S.A. (1996) Growth hormone and the insulin-like growth factor system in myogenesis. Endocrine Rev. 17, 481?517
16. Coleman, M.E., DeMayo, F., Yin, K.C., Lee, H.M., Geske, R., Montgomery, C., Schwartz. R.J. (1995) Myogenic vector expression of insulin-like growth factor 1 stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J. Biol. Chem. 270, 12109?12116
17. Jennische, E., Skottner, A., Hansson, H.A. (1987) Satellite cells express the trophic factor IGF-1 in regenerating skeletal muscle cell. Acta Physiol. Scand. 129, 9?15
18. Jannische, E., Hansson. H.A. (1987) Regenerating skeletal muscle cells express insulin-like growth factor I. Acta Physiol. Scand. 130, 327?332
- Joined
- Jul 25, 2008
- Messages
- 1,700
DAT, I ran across a thesis by Juha Hulmi you might like to look over.
Molecular and hormonal responses and adaptation to resistance exercise and protein nutrition in young and older men
**broken link removed**
Thanks for this BT.
It is a very interesting dissertation but is a lot of micro-analysis for little macro-practical recommendations.
The one solid general recommendation I have after reading a lot of this stuff is:
If you are an older person protein synthesis post-exercise is slower to begin. In order to insure a maximal response 3 grams of Leucine in PWO nutrition is recommended.
In fact in the elderly 3 grams of Leucine in PWO nutrition is needed to insure a solid protein synthesis response.
To find out the amount of individual amino acids in a certain foods goto: http://www.nutritiondata.com/
- Joined
- Jan 21, 2009
- Messages
- 648
In fact in the elderly 3 grams of Leucine in PWO nutrition is needed to insure a solid protein synthesis response.
I saw that. I have already started increasing the amount of BCCA I take PWO.
could just add the leucine  i had considered that for supplementation anyhow, just adding 500mg-1g of leucine with each meal. If you look at it, most animals have a different BCAA ratio than humans do, and it fall short on leucine so you rate limit everything else
i had considered that for supplementation anyhow, just adding 500mg-1g of leucine with each meal. If you look at it, most animals have a different BCAA ratio than humans do, and it fall short on leucine so you rate limit everything else
- Joined
- Jan 21, 2009
- Messages
- 648
could just add the leucinei had considered that for supplementation anyhow, just adding 500mg-1g of leucine with each meal. If you look at it, most animals have a different BCAA ratio than humans do, and it fall short on leucine so you rate limit everything else
I will probably do that once I use up this huge bottle of BCCA I have.
- Joined
- Jan 21, 2009
- Messages
- 648
Would fish oil work as well? or are the MCT's better for fat burning in the calorie restriction diet? Or maybe both?
MCTs, Energy and Exercise
MCTs provide about ten percent fewer calories than LCTs—8.3 calories per gram for MCTs versus 9 calories per gram for LCTs. But this is just one of the unique advantages of MCTs. More importantly, reduced chain length also means that MCTs are more rapidly absorbed by the body and more quickly metabolized (burned) as fuel (Fig. 1). The result of this accelerated metabolic conversion is that instead of being stored as fat, the calories contained in MCTs are very efficiently converted into fuel for immediate use by organs and muscles
MCTs provide about ten percent fewer calories than LCTs—8.3 calories per gram for MCTs versus 9 calories per gram for LCTs. But this is just one of the unique advantages of MCTs. More importantly, reduced chain length also means that MCTs are more rapidly absorbed by the body and more quickly metabolized (burned) as fuel (Fig. 1). The result of this accelerated metabolic conversion is that instead of being stored as fat, the calories contained in MCTs are very efficiently converted into fuel for immediate use by organs and muscles
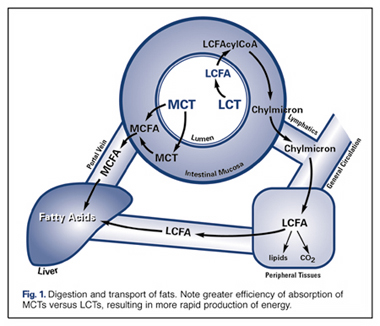
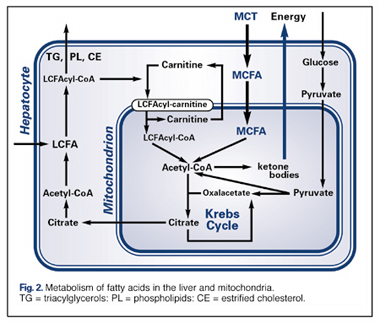
The energy-enhancing properties of MCTs are attributed to the fact that they cross the double mitochondrial membrane very rapidly, and do not require the presence of carnitine, as do LCTs (Fig. 2). The result is an excess of acetyl-coA, which then follows various metabolic pathways, both in the mitochondria (Krebs Cycle) and in the cytosol , resulting in the production of ketones . Scientists attribute the increased energy from consumption of MCTs to the rapid formation of ketone bodies. MCTs are thus a good choice for anyone who has increased energy needs, as following major surgery, during normal or stunted growth, to enhance athletic performance, and to counteract the decreased energy production that results from aging.
Other advantages:
1. MCTs are not stored in fat deposits in the body as much as LCTs.
2. MCTs have been shown to enhance thermogenesis.
3. MCTs behave metabolically in some fashion similar to carbohydrates, as well as their promoting the development of ketones
4. MCTs have been shown to suppress appetite.
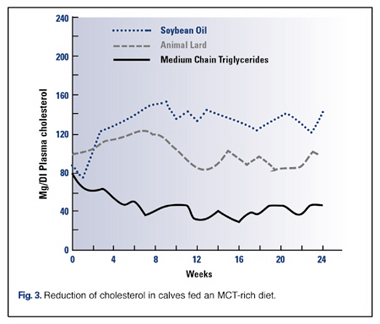
MCTs reduce levels of cholesterol in the liver and other tissuesMCTs have a number of properties that may be beneficial in preventing atherosclerosis by reducing levels of cholesterol in the liver and other tissues. MCTs have a slight hypoglycemic (blood glucose-lowering) effect, and thus may be useful for diabetics , or anyone with a tendency for pre-diabetes.
While fish oil or omega 3 are very good for our health, they are a very long chain triglyceride and not digested, absorbed and readily used for energy production like MTC's. So the answer to your question is MTC's are best.
Babayan, V.K. Medium chain fatty acid esters and their medical and nutritional applications. J Am Oil Chem Soc, 1981, 58: 49A-51A.
Kaunitz, H. Dietary use of MCT in “Bilanzierte Ernaehrung in der Therapie,” K. Lang, W. Fekl, and G. Berg, eds. George Thieme Verlag, Stuttgart, 1971.
Bach, A.C., and Babayan, V.K. Medium-chain triglycerides: An update. Am J Clin Nutr, 1982, 36: 950-962.
Stubbs RJ, Harbron CG. Covert manipulation of the ratio of medium- to long-chain triglycerides in isoenergetically dense diets: effect on food intake in ad libitum feeding men. Int J Obes Relat Metab Disord 1996 May;20(5):435-44.
Stewart, J.W., Wiggers, K.D., Jacobsen, N.L., Berger, P.J. Effect of various triglycerides on blood and tissue cholesterol of calves, J Nutr, 1978, 108: 561-566.
Kaunitz, H. Dietary use of MCT in “Bilanzierte Ernaehrung in der Therapie,” K. Lang, W. Fekl, and G. Berg, eds. George Thieme Verlag, Stuttgart, 1971.
Bach, A.C., and Babayan, V.K. Medium-chain triglycerides: An update. Am J Clin Nutr, 1982, 36: 950-962.
Stubbs RJ, Harbron CG. Covert manipulation of the ratio of medium- to long-chain triglycerides in isoenergetically dense diets: effect on food intake in ad libitum feeding men. Int J Obes Relat Metab Disord 1996 May;20(5):435-44.
Stewart, J.W., Wiggers, K.D., Jacobsen, N.L., Berger, P.J. Effect of various triglycerides on blood and tissue cholesterol of calves, J Nutr, 1978, 108: 561-566.
Last edited:
- Joined
- Jul 25, 2008
- Messages
- 1,700
Caprylic Acid (MCTs) - reduce expression of lipogenic genes
Thank you for posting that up BT. MCTs, specifically caprylic acid (octonate) does much more than that. Permit me to introduce you to one of my secrets. Very few people know and understand what follows. Best explained by the study Modulation of adipocyte lipogenesis by octanoate: involvement of reactive oxygen species, Wen Guo, Weisheng Xie and Jianrong Han, Nutrition & Metabolism 2006, 3:30
Background:
Medium-chain fatty acids (MCFA) belong to a unique type of fatty acids that is metabolized differently from either long-chain fatty acids or carbohydrates. Dietary Medium-chain triglycerides (MCT) inhibit body fat mass growth in both animals and human. Early studies suggest that this effect might be caused by rapid absorption of MCT-derived MCFA and their ß-oxidation in the liver, which reduces the circulating fatty acids available to the adipocytes [11]. This model is supported by the evidence that MCFA enters the ß-oxidation pathway in liver mitochondria independent of carnitine palmitoyl transferase I (CPT-I) [12].
[The afore-mentioned was presented by you BT & your wonderful illustrations]
However, it does not explain the findings that dietary MCT inhibits lipogenesis in adipocytes [13,14].
Furthermore, MCFA are recovered in the adipose tissue fatty acids up to 30 mole % in both animals and humans adapted to MCT diets [6,15-17]. These findings imply that a substantial influx of MCFA into the adipocytes occurs in vivo, which might affect adipose tissue function more than previously appreciated.
[MCTs in significant quantity do make there way into fat cells and do what?]
Indeed, we found that a reduction in fat mass was associated with reduced expression of lipogenic genes and adipocyte transcription factors in MCT-fed animals [6]. This effect was reproduced in cultured adipocytes treated with octanoate [18]. When added to differentiating rodent preadipocytes, MCFA also inhibits fat accumulation and reduces expression of adipocyte specific proteins [19,20]. In this study, we provide new evidence that octanoate suppresses lipogenesis, at least in part, by inactivating the key adipocyte transcription factor, peroxisome proliferator-activated receptor y (PPARy). Furthermore, our data revealed, for the first time, an involvement of reactive oxygen species (ROS) as a possible intermediate component that might regulate the anti-lipogenic effects.
[Wow!]
[What is so Wow?]
Discussion:
Fatty acid oxidation is normally activated only under fasting conditions when circulating levels of insulin and glucose are low. Conversely, lipogenesis is down-regulated by fasting. The mechanistic link between these two events, however, has not been established.
In this work, we provided the first evidence that medium-chain octanoate can be ß-oxidized in adipocytes independent of CPT-I regulation. Hence, supplement of octanoate maintains active ß- oxidation in the presence of insulin and glucose. This is correlated with inhibition of lipogenesis and reduction of lipogenic gene expression. In other words, octanoate induces a metabolic state in adipocytes mimicking a fasting condition without actual hormone/nutrient deprivation. Our results also demonstrated that ROS might be involved as a mediator for octanoate in lowering PPARy activity, the master control of lipogenic gene expression.
As extensively reviewed previously, PPARy is a prototypical member of the nuclear receptor superfamily which integrates the control of energy, lipid and glucose, homerostasis [50-54]. PPARy binds a variety of small lipophilic compounds derived from metabolism and nutrition. These ligands, in turn, determine cofactor recruitment and regulate the transcription of a variety of metabolic genes. Recent literature highlights the development of partial agonists of PPARy to block adipogenesis and reduce fat mass development [54-59]. In one of our previous studies, we proposed that octanoate might act as a partial agonist for PPARy because it can potentially bind to PPARy as does the long-chain fatty acids [29,60], hence competitively blocking the binding of the latter or other endogenous ligands. This model was supported, but not proved, by the findings that the anti-adipogenic [19] and antilipogenic (this work) effects of octanoate was efficiently blocked by selected synthetic PPARy agonists.
The current findings that octanoate induced ROS generation in adipocytes suggest that octanoate might also modulate PPARy activity indirectly via the ROS signaling pathways. It has been well established that ROS activates the stress-responsive protein kinases [61,62], which either directly or indirectly inhibit PPARy activity [47-49,62-67]. In our preliminary studies, we found that octanoate also induced sustained activation of Erk1/2 and JNK/SAPK (data not shown). How these kinase pathways are involved in the regulation of PPARy activity and lipogenesis in our cell system and, more importantly, in primary adipocytes, are currently under investigation.
Inhibition of adipocyte lipogenesis can be a useful tool for the prevention of obesity. In this regard, our studies contribute to the scientific basis for the application of MCT in dietary weight management. On the other hand, a complete inhibition of fat mass growth is disastrous since adipocytes play important roles in physiological functions of mammals. Compared to the pharmaceutical inhibitors of lipogenesis [68,69], the effects of octanoate can be considered as moderate and yet might be more desirable for physiological regulation of body fat mass without adversely affecting normal fat tissue functions. According to recent surveys, a majority of the middle age population is moderately over-weighed (BMI 23–27), and a slight increase in BMI in this range is associated with a greater risk for metabolic syndrome [70,71]. It will be of important social and economical values if MCT can be used for body weight regulation in this sub-population, as demonstrated by a recent clinical trial [5].
Conclusion:
This study demonstrated that octanoate had a direct inhibitory effect on fat storage in adipocytes under conditions that normally favor lipogenesis. This was related to its unique ß-oxidation mechanism which links to elevated cellular ROS levels and subsequent inactivation of PPARy. The exact mechanism by which PPARy is inactivated, in particular, how ROS is involved in this process, still remains to be elucidated. Furthermore, ROS is known to have diverse and complex molecular targets, which might directly or indirectly influence the activities of additional adipocyte transcription factors or modify selected lipogenic proteins [44,71]. Elucidation of these mechanisms will be helpful for the application of MCT for dietary intervention to prevent obesity and may reveal possible pharmaceutical targets to modulate fat metabolism.
From the RESULTS an important practical tip [L-Carnitine & MCTs (Caprylic Acid) should not be used together & MCTs (Caprylic Acid) are more effective then L-Carnitine in the presence of insulin]
As shown in Figure 3A, ß-oxidation of octanoate was slightly inhibited (~18%) by insulin, a hormone that promotes the generation of the natural inhibitor of CPT-I [37], and Etomoxir, a pharmaceutical inhibitor of CPT-I. On the other hand, L-carnitine, an activator of CPT-I, caused a ~60% inhibition of octanoate oxidation. A combination of L-carnitine and exogenous oleate further enhanced the inhibition (> 85%). In contrast, ß-oxidation of oleate was increased by L-carnitine more than 2 fold but inhibited by insulin by about 60% (Fig. 3B), consistent with the literature [37]. These results indicate that in adipocytes, octanoate was mainly oxidized independent of CPT-I (> 80%). A small fraction (< 20%), that was sensitive to insulin and etomoxir, might be activated in the cytosol and hence depend on CPT-I to enter the mitochondria. The observation that L-carnitine inhibited, rather than promoted, ß-oxidation of octanoate suggests that activation of CPT-I largely increased the transport of endogenous fatty acids into the ß-oxidation pathway which compete with octanoate for the enzymes downstream from CPT-1. This competition was further enhanced in the presence of added oleate.
Thank you for posting that up BT. MCTs, specifically caprylic acid (octonate) does much more than that. Permit me to introduce you to one of my secrets. Very few people know and understand what follows. Best explained by the study Modulation of adipocyte lipogenesis by octanoate: involvement of reactive oxygen species, Wen Guo, Weisheng Xie and Jianrong Han, Nutrition & Metabolism 2006, 3:30
Background:
Medium-chain fatty acids (MCFA) belong to a unique type of fatty acids that is metabolized differently from either long-chain fatty acids or carbohydrates. Dietary Medium-chain triglycerides (MCT) inhibit body fat mass growth in both animals and human. Early studies suggest that this effect might be caused by rapid absorption of MCT-derived MCFA and their ß-oxidation in the liver, which reduces the circulating fatty acids available to the adipocytes [11]. This model is supported by the evidence that MCFA enters the ß-oxidation pathway in liver mitochondria independent of carnitine palmitoyl transferase I (CPT-I) [12].
[The afore-mentioned was presented by you BT & your wonderful illustrations]
However, it does not explain the findings that dietary MCT inhibits lipogenesis in adipocytes [13,14].
Furthermore, MCFA are recovered in the adipose tissue fatty acids up to 30 mole % in both animals and humans adapted to MCT diets [6,15-17]. These findings imply that a substantial influx of MCFA into the adipocytes occurs in vivo, which might affect adipose tissue function more than previously appreciated.
[MCTs in significant quantity do make there way into fat cells and do what?]
Indeed, we found that a reduction in fat mass was associated with reduced expression of lipogenic genes and adipocyte transcription factors in MCT-fed animals [6]. This effect was reproduced in cultured adipocytes treated with octanoate [18]. When added to differentiating rodent preadipocytes, MCFA also inhibits fat accumulation and reduces expression of adipocyte specific proteins [19,20]. In this study, we provide new evidence that octanoate suppresses lipogenesis, at least in part, by inactivating the key adipocyte transcription factor, peroxisome proliferator-activated receptor y (PPARy). Furthermore, our data revealed, for the first time, an involvement of reactive oxygen species (ROS) as a possible intermediate component that might regulate the anti-lipogenic effects.
[Wow!]
[What is so Wow?]
Discussion:
Fatty acid oxidation is normally activated only under fasting conditions when circulating levels of insulin and glucose are low. Conversely, lipogenesis is down-regulated by fasting. The mechanistic link between these two events, however, has not been established.
In this work, we provided the first evidence that medium-chain octanoate can be ß-oxidized in adipocytes independent of CPT-I regulation. Hence, supplement of octanoate maintains active ß- oxidation in the presence of insulin and glucose. This is correlated with inhibition of lipogenesis and reduction of lipogenic gene expression. In other words, octanoate induces a metabolic state in adipocytes mimicking a fasting condition without actual hormone/nutrient deprivation. Our results also demonstrated that ROS might be involved as a mediator for octanoate in lowering PPARy activity, the master control of lipogenic gene expression.
As extensively reviewed previously, PPARy is a prototypical member of the nuclear receptor superfamily which integrates the control of energy, lipid and glucose, homerostasis [50-54]. PPARy binds a variety of small lipophilic compounds derived from metabolism and nutrition. These ligands, in turn, determine cofactor recruitment and regulate the transcription of a variety of metabolic genes. Recent literature highlights the development of partial agonists of PPARy to block adipogenesis and reduce fat mass development [54-59]. In one of our previous studies, we proposed that octanoate might act as a partial agonist for PPARy because it can potentially bind to PPARy as does the long-chain fatty acids [29,60], hence competitively blocking the binding of the latter or other endogenous ligands. This model was supported, but not proved, by the findings that the anti-adipogenic [19] and antilipogenic (this work) effects of octanoate was efficiently blocked by selected synthetic PPARy agonists.
The current findings that octanoate induced ROS generation in adipocytes suggest that octanoate might also modulate PPARy activity indirectly via the ROS signaling pathways. It has been well established that ROS activates the stress-responsive protein kinases [61,62], which either directly or indirectly inhibit PPARy activity [47-49,62-67]. In our preliminary studies, we found that octanoate also induced sustained activation of Erk1/2 and JNK/SAPK (data not shown). How these kinase pathways are involved in the regulation of PPARy activity and lipogenesis in our cell system and, more importantly, in primary adipocytes, are currently under investigation.
Inhibition of adipocyte lipogenesis can be a useful tool for the prevention of obesity. In this regard, our studies contribute to the scientific basis for the application of MCT in dietary weight management. On the other hand, a complete inhibition of fat mass growth is disastrous since adipocytes play important roles in physiological functions of mammals. Compared to the pharmaceutical inhibitors of lipogenesis [68,69], the effects of octanoate can be considered as moderate and yet might be more desirable for physiological regulation of body fat mass without adversely affecting normal fat tissue functions. According to recent surveys, a majority of the middle age population is moderately over-weighed (BMI 23–27), and a slight increase in BMI in this range is associated with a greater risk for metabolic syndrome [70,71]. It will be of important social and economical values if MCT can be used for body weight regulation in this sub-population, as demonstrated by a recent clinical trial [5].
Conclusion:
This study demonstrated that octanoate had a direct inhibitory effect on fat storage in adipocytes under conditions that normally favor lipogenesis. This was related to its unique ß-oxidation mechanism which links to elevated cellular ROS levels and subsequent inactivation of PPARy. The exact mechanism by which PPARy is inactivated, in particular, how ROS is involved in this process, still remains to be elucidated. Furthermore, ROS is known to have diverse and complex molecular targets, which might directly or indirectly influence the activities of additional adipocyte transcription factors or modify selected lipogenic proteins [44,71]. Elucidation of these mechanisms will be helpful for the application of MCT for dietary intervention to prevent obesity and may reveal possible pharmaceutical targets to modulate fat metabolism.
From the RESULTS an important practical tip [L-Carnitine & MCTs (Caprylic Acid) should not be used together & MCTs (Caprylic Acid) are more effective then L-Carnitine in the presence of insulin]
As shown in Figure 3A, ß-oxidation of octanoate was slightly inhibited (~18%) by insulin, a hormone that promotes the generation of the natural inhibitor of CPT-I [37], and Etomoxir, a pharmaceutical inhibitor of CPT-I. On the other hand, L-carnitine, an activator of CPT-I, caused a ~60% inhibition of octanoate oxidation. A combination of L-carnitine and exogenous oleate further enhanced the inhibition (> 85%). In contrast, ß-oxidation of oleate was increased by L-carnitine more than 2 fold but inhibited by insulin by about 60% (Fig. 3B), consistent with the literature [37]. These results indicate that in adipocytes, octanoate was mainly oxidized independent of CPT-I (> 80%). A small fraction (< 20%), that was sensitive to insulin and etomoxir, might be activated in the cytosol and hence depend on CPT-I to enter the mitochondria. The observation that L-carnitine inhibited, rather than promoted, ß-oxidation of octanoate suggests that activation of CPT-I largely increased the transport of endogenous fatty acids into the ß-oxidation pathway which compete with octanoate for the enzymes downstream from CPT-1. This competition was further enhanced in the presence of added oleate.
References:
1. Bray GA, Lee M, Bray TL: Weight gain of rats fed medium-chain triglycerides is less than rats fed long-chain triglycerides. Int J Obes 1980, 4:27-32.
2. Hashim SA, Tantibhedyangkul P: Medium chain triglyceride in early life: effects on growth of adipose tissue. Lipids 1987, 22:429-434.
3. Papamandjaris AA, White MD, Raeini-Sarjaz M, Jones PJ: Endogenous fat oxidation during medium chain versus long chain triglyceride feeding in healthy women. Int J Obes Relat Metab Disord 2000, 24:1158-1166.
4. Tsuji H, Kasai M, Takeuchi H, Nakamura M, Okazaki M, Kondo K: Dietary medium-chain triacylglycerols suppress accumulation of body fat in a double-blind, controlled trial in healthy men and women. J Nutr 2001, 131:2853-2859.
5. Nosaka N, Maki H, Suzuki Y, Haruna H, Ohara A, Kasai M, Tsuji H, Aoyama T, Okazaki M, Igarashi O, Kondo K: Effects of margarine containing medium-chain triacylglycerols on body fat reduction in humans. J Atheroscler Thromb 2003, 10:290-298.
6. Han J, Hamilton JA, Kirkland JL, Corkey BE, Guo W: Medium-chain oil reduces fat mass and down-regulates expression of adipogenic genes in rats. Obes Res 2003, 11:734-744.
7. St-Onge MP, Ross R, Parsons WD, Jones PJ: Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes Res 2003, 11:395-402.
8. St-Onge MP, Jones PJ: Greater rise in fat oxidation with medium-chain triglyceride consumption relative to longchain triglyceride is associated with lower initial body weight and greater loss of subcutaneous adipose tissue. Int J Obes Relat Metab Disord 2003, 27:1565-1571.
9. Bourque C, St-Onge MP, Papamandjaris AA, Cohn JS, Jones PJ: Consumption of an oil composed of medium chain triacyglycerols, phytosterols, and N-3 fatty acids improves cardiovascular risk profile in overweight women. Metabolism 2003, 52:771-777.
10. St-Onge MP, Bourque C, Jones PJ, Ross R, Parsons WE: Mediumversus long-chain triglycerides for 27 days increases fat oxidation and energy expenditure without resulting in changes in body composition in overweight women. Int J Obes Relat Metab Disord 2003, 27:95-102.
11. Bach AC, Ingenbleek Y, Frey A: The usefulness of dietary medium-chain triglycerides in body weight control: fact or fancy? J Lipid Res 1996, 37:708-726.
12. Aas M: Organ and subcellular distribution of fatty acid activating enzymes in the rat. Biochim Biophys Acta 1971, 231:32-47.
13. Wiley JH, Leveille GA: Metabolic consequences of dietary medium-chain triglycerides in the rat. J Nutr 1973, 103:829-835.
14. Lavau MM, Hashim SA: Effect of medium chain triglyceride on lipogenesis and body fat in the rat. J Nutr 1978, 108:613-620.
15. Hill JO, Peters JC, Lin D, Yakubu F, Greene H, Swift L: Lipid accumulation and body fat distribution is influenced by type of dietary fat fed to rats. Int J Obes Relat Metab Disord 1993, 17:223-236.
16. Kinkela T, Chanussot F, Bach A, Max JP, Schirardin H, Debry G: Effects of diets containing medium-chain and long-chain triacylglycerols in the genetically obese Zucker fa/fa rat. Composition of fatty acids and triacylglycerols of the liver and adipose tissues. Ann Nutr Metab 1983, 27:404-414.
17. Sarda P, Lepage G, Roy CC, Chessex P: Storage of medium-chain triglycerides in adipose tissue of orally fed infants. Am J Clin Nutr 1987, 45:399-405.
18. Guo W, Lei T, Wang T, Corkey BE, Han J: Octanoate inhibits triglyceride synthesis in 3T3-L1 and human adipocytes. J Nutr 2003, 133:2512-2518.
19. Han J, Farmer SR, Kirkland JL, Corkey BE, Yoon R, Pirtskhalava T, Ido Y, Guo W: Octanoate attenuates adipogenesis in 3T3-L1 preadipocytes. J Nutr 2002, 132:904-910.
20. Nakajima I, Muroya S, Chikuni K: Growth arrest by octanoate is required for porcine preadipocyte differentiation. Biochem Biophys Res Commun 2003, 309:702-708.
21. Graves RA, Tontonoz P, Spiegelman BM: Analysis of a tissue-specific enhancer: ARF6 regulates adipogenic gene expression. Mol Cell Biol 1992, 12:1202-1208.
22. Guo W, Choi JK, Kirkland JL, Corkey BE, Hamilton JA: Esterification of free fatty acids in adipocytes: a comparison between octanoate and oleate. Biochem J 2000, 349:463-471.
23. Wang T, Zang Y, Ling W, Corkey BE, Guo W: Metabolic partitioning of endogenous fatty acid in adipocytes. Obes Res 2003, 11:880-887.
24. Guo WLT, Wang T, Corkey BE, Han J: Octanoate inhibits triglyceride synthesis in 3T3-L1 and human adipocytes. J Nutr 2003, 133:2512-2518.
25. Talior I, Yarkoni M, Bashan N, Eldar-Finkelman H: Increased glucose uptake promotes oxidative stress and PKC-delta activation in adipocytes of obese, insulin-resistant mice. Am J Physiol Endocrinol Metab 2003, 285:E295-302.
26. Hajri T, Han XX, Bonen A, Abumrad NA: Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest 2002, 109:1381-1389.
27. Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, Staels B, Auwerx J: PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J 15(19):5336-48. 1996 Oct 1
28. Sato O, Kuriki C, Fukui Y, Motojima K: Dual promoter structure of mouse and human fatty acid translocase/CD36 genes and unique transcriptional activation by peroxisome proliferator- activated receptor alpha and gamma ligands. J Biol Chem 2002, 277(18):15703-11. Epub 2002 Feb 26.
29. Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM: Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA 1997, 94:4318-4323.
30. Shillabeer G, Lau DC: Regulation of new fat cell formation in rats: the role of dietary fats. J Lipid Res 1994, 35:592-600. 31. Shillabeer G, Forden JM, Lau DC: Induction of preadipocyte differentiation by mature fat cells in the rat. J Clin Invest 1989, 84:381-387.
32. Brandes R, Arad R, Bar-Tana J: Inducers of adipose conversion activate transcription promoted by a peroxisome proliferators response element in 3T3-L1 cells. Biochem Pharmacol 1995, 50:1949-1951.
33. Ibrahimi A, Teboul L, Gaillard D, Amri EZ, Ailhaud G, Young P, Cawthorne MA, Grimaldi PA: Evidence for a common mechanism of action for fatty acids and thiazolidinedione antidiabetic agents on gene expression in preadipose cells. Mol Pharmacol 1994, 46:1070-1076.
34. Ailhaud G, Amri EZ, Grimaldi PA: Fatty acids and expression of lipid-related genes in adipose cells. Proc Nutr Soc 1996, 55:151-154.
35. Ailhaud G, Amri EZ, Grimaldi PA: Fatty acids and adipose cell differentiation. Prostaglandins Leukot Essent Fatty Acids 1995, 52:113-115.
36. Amri EZ, Bertrand B, Ailhaud G, Grimaldi P: Regulation of adipose cell differentiation. I. Fatty acids are inducers of the aP2 gene expression. J Lipid Res 1991, 32:1449-1456.
37. McGarry JD, Sen A, Esser V, Woeltje KF, Weis B, Foster DW: New insights into the mitochondrial carnitine palmitoyltransferase enzyme system. Biochimie 1991, 73:77-84.
38. Fujino T, Takei YA, Sone H, Ioka RX, Kamataki A, Magoori K, Takahashi S, Sakai J, Yamamoto TT: Molecular identification and characterization of two medium-chain acyl-CoA synthetases, MACS1 and the Sa gene product. J Biol Chem 2001, 276:35961-35966.
39. Vessey DA, Lau E, Kelley M, Warren RS: Isolation, sequencing, and expression of a cDNA for the HXM-A form of xenobiotic/ medium-chain fatty acid:CoA ligase from human liver mitochondria. J Biochem Mol Toxicol 2003, 17:1-6.
40. Oka Y, Kobayakawa K, Nishizumi H, Miyamichi K, Hirose S, Tsuboi A, Sakano H: O-MACS, a novel member of the medium-chain acyl-CoA synthetase family, specifically expressed in the olfactory epithelium in a zone-specific manner. Eur J Biochem 2003, 270:1995-2004.
41. Lei T, Xie W, Watkins PA, Guo W: Activation of medium-chain fatty acids in 3T3-L1 adipocytes and mouse adipose tissue. Obes Res 2003, 11:180P. (abstract)
42. Hickson-Bick DL, Sparagna GC, Buja LM, McMillin JB: Palmitateinduced apoptosis in neonatal cardiomyocytes is not dependent on the generation of ROS. Am J Physiol Heart Circ Physiol 2002, 282:H656-664.
43. Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M: Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem 2001, 276:25096-25100.
44. Yamagishi S, Okamoto T, Amano S, Inagaki Y, Koga K, Koga M, Choei H, Sasaki N, Kikuchi S, Takeuchi M, Makita Z: Palmitate-induced apoptosis of microvascular endothelial cells and pericytes. Mol Med 2002, 8:179-184.
45. Turrens JF, Alexandre A, Lehninger AL: Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 1985, 237:408-414.
46. Young TA, Cunningham CC, Bailey SM: Reactive oxygen species production by the mitochondrial respiratory chain in isolated rat hepatocytes and liver mitochondria: studies using myxothiazol. Arch Biochem Biophys 2002, 140:65-72.
47. Hu E, Kim JB, Sarraf P, Spiegelman BM: Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 1996, 274:2100-2103.
48. Yang W, Hong YH, Shen XQ, Frankowski C, Camp HS, Leff T: Regulation of transcription by AMP-activated protein kinase: phosphorylation of p300 blocks its interaction with nuclear receptors. J Biol Chem 2001, 276:38341-38344.
49. Camp HS, Tafuri SR, Leff T: c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology 1999, 140:392-397.
50. Debril MB, Renaud JP, Fajas L, Auwerx J: The pleiotropic functions of peroxisome proliferator-activated receptor gamma. J Mol Med 2001, 79:30-47.
51. Rosen ED, Spiegelman BM: PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem 2001, 276:37731-37734.
52. Hihi AK, Michalik L, Wahli W: PPARs: transcriptional effectors of fatty acids and their derivatives. Cell Mol Life Sci 2002, 59:790-798.
53. Sewter C, Vidal-Puig A: PPARgamma and the thiazolidinediones: molecular basis for a treatment of 'Syndrome X'? Diabetes Obes Metab 2002, 4:239-248.
54. Knouff C, Auwerx J: Peroxisome proliferator-activated receptor- gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev 2004, 25:899-918.
55. Oberfield JL, Collins JL, Holmes CP, Goreham DM, Cooper JP, Cobb JE, Lenhard JM, Hull-Ryde EA, Mohr CP, Blanchard SG, Parks DJ, Moore LB, Lehmann JM, Plunket K, Miller AB, Milburn MV, Kliewer SA, Willson TM: A peroxisome proliferator-activated receptor gamma ligand inhibits adipocyte differentiation. Proc Natl Acad Sci U S A 1999, 96:6102-6106.
56. Mukherjee R, Hoener PA, Jow L, Bilakovics J, Klausing K, Mais DE, Faulkner A, Croston GE, Paterniti JR Jr: A selective peroxisome proliferator-activated receptor-gamma (PPARgamma) modulator blocks adipocyte differentiation but stimulates glucose uptake in 3T3-L1 adipocytes. Mol Endocrinol 2000, 14:1425-1433.
57. Camp HS, Chaudhry A, Leff T: A novel potent antagonist of peroxisome proliferator-activated receptor gamma blocks adipocyte differentiation but does not revert the phenotype of terminally differentiated adipocytes. Endocrinology 2001, 142:3207-3213.
58. Lee G, Elwood F, McNally J, Weiszmann J, Lindstrom M, Amaral K, Nakamura M, Miao S, Cao P, Learned RM, Chen JL, Li Y: T007 a selective ligand for peroxisome proliferator-activated receptor gamma, functions as an antagonist of biochemical and cellular activities. J Biol Chem 0907, 277:19649-19657.
59. Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, Davis RG, Hull-Ryde EA, Lenhard JM, Patel L, Plunket KD, Shenk JL, Stimmel JB, Therapontos C, Willson TM, Blanchard SG: Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 2002, 28:6640-6650.
60. Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV: Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell 1999, 3:397-403.
61. Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, Soo Kim S, Ha J: The regulation of AMP-activated protein kinase by H(2)O(2). Biochem Biophys Res Commun 2001, 287:92-97.
62. Souza SC, Palmer HJ, Kang YH, Yamamoto MT, Muliro KV, Paulson KE, Greenberg AS: TNF-alpha induction of lipolysis is mediated through activation of the extracellular signal related kinase pathway in 3T3-L1 adipocytes. J Cell Biochem 2003, 89:1077-1086.
63. Floyd ZE, Stephens JM: Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem 2002, 277:4062-4068.
64. Huang WC, Chio CC, Chi KH, Wu HM, Lin WW: Superoxide anion-dependent Raf/MEK/ERK activation by peroxisome proliferator activated receptor gamma agonists 15-deoxydelta( 12,14)-prostaglandin J(2), ciglitazone, and GW1929. Exp Cell Res 2002, 277:192-200.
65. Hedvat M, Jain A, Carson DA, Leoni LM, Huang G, Holden S, Lu D, Corr M, Fox W, Agus DB: Inhibition of HER-kinase activation prevents ERK-mediated degradation of PPARgamma. Cancer Cell 2004, 5:565-574.
66. Tanabe Y, Nakayama K: Mechanical stretching inhibits adipocyte differentiation of 3T3-L1 cells: the molecular mechanism and pharmacological regulation. Nippon Yakurigaku Zasshi 2004, 124:337-344.
67. Tanabe Y, Koga M, Saito M, Matsunaga Y, Nakayama K: Inhibition of adipocyte differentiation by mechanical stretching through ERK-mediated downregulation of PPARgamma2. J Cell Sci 2004, 117:3605-3614.
68. Goransson O, Ryden M, Nilsson R, Arner P, Degerman E: Dimethylaminopurine inhibits metabolic effects of insulin in primary adipocytes. In J Nutr Biochem 2004:303-312.
69. Thupari JN, Landree LE, Ronnett GV, Kuhajda FP: C75 increases peripheral energy utilization and fatty acid oxidation in dietinduced obesity. Proc Natl Acad Sci U S A 2002, 99:9498-9502.
70. St-Onge MP: Relationship between body composition changes and changes in physical function and metabolic risk factors in aging. Curr Opin Clin Nutr Metab Care 2005, 8:523-528.
71. St-Onge MP, Janssen I, Heymsfield SB: Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care 2004, 27:2222-2228.
1. Bray GA, Lee M, Bray TL: Weight gain of rats fed medium-chain triglycerides is less than rats fed long-chain triglycerides. Int J Obes 1980, 4:27-32.
2. Hashim SA, Tantibhedyangkul P: Medium chain triglyceride in early life: effects on growth of adipose tissue. Lipids 1987, 22:429-434.
3. Papamandjaris AA, White MD, Raeini-Sarjaz M, Jones PJ: Endogenous fat oxidation during medium chain versus long chain triglyceride feeding in healthy women. Int J Obes Relat Metab Disord 2000, 24:1158-1166.
4. Tsuji H, Kasai M, Takeuchi H, Nakamura M, Okazaki M, Kondo K: Dietary medium-chain triacylglycerols suppress accumulation of body fat in a double-blind, controlled trial in healthy men and women. J Nutr 2001, 131:2853-2859.
5. Nosaka N, Maki H, Suzuki Y, Haruna H, Ohara A, Kasai M, Tsuji H, Aoyama T, Okazaki M, Igarashi O, Kondo K: Effects of margarine containing medium-chain triacylglycerols on body fat reduction in humans. J Atheroscler Thromb 2003, 10:290-298.
6. Han J, Hamilton JA, Kirkland JL, Corkey BE, Guo W: Medium-chain oil reduces fat mass and down-regulates expression of adipogenic genes in rats. Obes Res 2003, 11:734-744.
7. St-Onge MP, Ross R, Parsons WD, Jones PJ: Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes Res 2003, 11:395-402.
8. St-Onge MP, Jones PJ: Greater rise in fat oxidation with medium-chain triglyceride consumption relative to longchain triglyceride is associated with lower initial body weight and greater loss of subcutaneous adipose tissue. Int J Obes Relat Metab Disord 2003, 27:1565-1571.
9. Bourque C, St-Onge MP, Papamandjaris AA, Cohn JS, Jones PJ: Consumption of an oil composed of medium chain triacyglycerols, phytosterols, and N-3 fatty acids improves cardiovascular risk profile in overweight women. Metabolism 2003, 52:771-777.
10. St-Onge MP, Bourque C, Jones PJ, Ross R, Parsons WE: Mediumversus long-chain triglycerides for 27 days increases fat oxidation and energy expenditure without resulting in changes in body composition in overweight women. Int J Obes Relat Metab Disord 2003, 27:95-102.
11. Bach AC, Ingenbleek Y, Frey A: The usefulness of dietary medium-chain triglycerides in body weight control: fact or fancy? J Lipid Res 1996, 37:708-726.
12. Aas M: Organ and subcellular distribution of fatty acid activating enzymes in the rat. Biochim Biophys Acta 1971, 231:32-47.
13. Wiley JH, Leveille GA: Metabolic consequences of dietary medium-chain triglycerides in the rat. J Nutr 1973, 103:829-835.
14. Lavau MM, Hashim SA: Effect of medium chain triglyceride on lipogenesis and body fat in the rat. J Nutr 1978, 108:613-620.
15. Hill JO, Peters JC, Lin D, Yakubu F, Greene H, Swift L: Lipid accumulation and body fat distribution is influenced by type of dietary fat fed to rats. Int J Obes Relat Metab Disord 1993, 17:223-236.
16. Kinkela T, Chanussot F, Bach A, Max JP, Schirardin H, Debry G: Effects of diets containing medium-chain and long-chain triacylglycerols in the genetically obese Zucker fa/fa rat. Composition of fatty acids and triacylglycerols of the liver and adipose tissues. Ann Nutr Metab 1983, 27:404-414.
17. Sarda P, Lepage G, Roy CC, Chessex P: Storage of medium-chain triglycerides in adipose tissue of orally fed infants. Am J Clin Nutr 1987, 45:399-405.
18. Guo W, Lei T, Wang T, Corkey BE, Han J: Octanoate inhibits triglyceride synthesis in 3T3-L1 and human adipocytes. J Nutr 2003, 133:2512-2518.
19. Han J, Farmer SR, Kirkland JL, Corkey BE, Yoon R, Pirtskhalava T, Ido Y, Guo W: Octanoate attenuates adipogenesis in 3T3-L1 preadipocytes. J Nutr 2002, 132:904-910.
20. Nakajima I, Muroya S, Chikuni K: Growth arrest by octanoate is required for porcine preadipocyte differentiation. Biochem Biophys Res Commun 2003, 309:702-708.
21. Graves RA, Tontonoz P, Spiegelman BM: Analysis of a tissue-specific enhancer: ARF6 regulates adipogenic gene expression. Mol Cell Biol 1992, 12:1202-1208.
22. Guo W, Choi JK, Kirkland JL, Corkey BE, Hamilton JA: Esterification of free fatty acids in adipocytes: a comparison between octanoate and oleate. Biochem J 2000, 349:463-471.
23. Wang T, Zang Y, Ling W, Corkey BE, Guo W: Metabolic partitioning of endogenous fatty acid in adipocytes. Obes Res 2003, 11:880-887.
24. Guo WLT, Wang T, Corkey BE, Han J: Octanoate inhibits triglyceride synthesis in 3T3-L1 and human adipocytes. J Nutr 2003, 133:2512-2518.
25. Talior I, Yarkoni M, Bashan N, Eldar-Finkelman H: Increased glucose uptake promotes oxidative stress and PKC-delta activation in adipocytes of obese, insulin-resistant mice. Am J Physiol Endocrinol Metab 2003, 285:E295-302.
26. Hajri T, Han XX, Bonen A, Abumrad NA: Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest 2002, 109:1381-1389.
27. Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, Staels B, Auwerx J: PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J 15(19):5336-48. 1996 Oct 1
28. Sato O, Kuriki C, Fukui Y, Motojima K: Dual promoter structure of mouse and human fatty acid translocase/CD36 genes and unique transcriptional activation by peroxisome proliferator- activated receptor alpha and gamma ligands. J Biol Chem 2002, 277(18):15703-11. Epub 2002 Feb 26.
29. Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM: Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA 1997, 94:4318-4323.
30. Shillabeer G, Lau DC: Regulation of new fat cell formation in rats: the role of dietary fats. J Lipid Res 1994, 35:592-600. 31. Shillabeer G, Forden JM, Lau DC: Induction of preadipocyte differentiation by mature fat cells in the rat. J Clin Invest 1989, 84:381-387.
32. Brandes R, Arad R, Bar-Tana J: Inducers of adipose conversion activate transcription promoted by a peroxisome proliferators response element in 3T3-L1 cells. Biochem Pharmacol 1995, 50:1949-1951.
33. Ibrahimi A, Teboul L, Gaillard D, Amri EZ, Ailhaud G, Young P, Cawthorne MA, Grimaldi PA: Evidence for a common mechanism of action for fatty acids and thiazolidinedione antidiabetic agents on gene expression in preadipose cells. Mol Pharmacol 1994, 46:1070-1076.
34. Ailhaud G, Amri EZ, Grimaldi PA: Fatty acids and expression of lipid-related genes in adipose cells. Proc Nutr Soc 1996, 55:151-154.
35. Ailhaud G, Amri EZ, Grimaldi PA: Fatty acids and adipose cell differentiation. Prostaglandins Leukot Essent Fatty Acids 1995, 52:113-115.
36. Amri EZ, Bertrand B, Ailhaud G, Grimaldi P: Regulation of adipose cell differentiation. I. Fatty acids are inducers of the aP2 gene expression. J Lipid Res 1991, 32:1449-1456.
37. McGarry JD, Sen A, Esser V, Woeltje KF, Weis B, Foster DW: New insights into the mitochondrial carnitine palmitoyltransferase enzyme system. Biochimie 1991, 73:77-84.
38. Fujino T, Takei YA, Sone H, Ioka RX, Kamataki A, Magoori K, Takahashi S, Sakai J, Yamamoto TT: Molecular identification and characterization of two medium-chain acyl-CoA synthetases, MACS1 and the Sa gene product. J Biol Chem 2001, 276:35961-35966.
39. Vessey DA, Lau E, Kelley M, Warren RS: Isolation, sequencing, and expression of a cDNA for the HXM-A form of xenobiotic/ medium-chain fatty acid:CoA ligase from human liver mitochondria. J Biochem Mol Toxicol 2003, 17:1-6.
40. Oka Y, Kobayakawa K, Nishizumi H, Miyamichi K, Hirose S, Tsuboi A, Sakano H: O-MACS, a novel member of the medium-chain acyl-CoA synthetase family, specifically expressed in the olfactory epithelium in a zone-specific manner. Eur J Biochem 2003, 270:1995-2004.
41. Lei T, Xie W, Watkins PA, Guo W: Activation of medium-chain fatty acids in 3T3-L1 adipocytes and mouse adipose tissue. Obes Res 2003, 11:180P. (abstract)
42. Hickson-Bick DL, Sparagna GC, Buja LM, McMillin JB: Palmitateinduced apoptosis in neonatal cardiomyocytes is not dependent on the generation of ROS. Am J Physiol Heart Circ Physiol 2002, 282:H656-664.
43. Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M: Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem 2001, 276:25096-25100.
44. Yamagishi S, Okamoto T, Amano S, Inagaki Y, Koga K, Koga M, Choei H, Sasaki N, Kikuchi S, Takeuchi M, Makita Z: Palmitate-induced apoptosis of microvascular endothelial cells and pericytes. Mol Med 2002, 8:179-184.
45. Turrens JF, Alexandre A, Lehninger AL: Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 1985, 237:408-414.
46. Young TA, Cunningham CC, Bailey SM: Reactive oxygen species production by the mitochondrial respiratory chain in isolated rat hepatocytes and liver mitochondria: studies using myxothiazol. Arch Biochem Biophys 2002, 140:65-72.
47. Hu E, Kim JB, Sarraf P, Spiegelman BM: Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 1996, 274:2100-2103.
48. Yang W, Hong YH, Shen XQ, Frankowski C, Camp HS, Leff T: Regulation of transcription by AMP-activated protein kinase: phosphorylation of p300 blocks its interaction with nuclear receptors. J Biol Chem 2001, 276:38341-38344.
49. Camp HS, Tafuri SR, Leff T: c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology 1999, 140:392-397.
50. Debril MB, Renaud JP, Fajas L, Auwerx J: The pleiotropic functions of peroxisome proliferator-activated receptor gamma. J Mol Med 2001, 79:30-47.
51. Rosen ED, Spiegelman BM: PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem 2001, 276:37731-37734.
52. Hihi AK, Michalik L, Wahli W: PPARs: transcriptional effectors of fatty acids and their derivatives. Cell Mol Life Sci 2002, 59:790-798.
53. Sewter C, Vidal-Puig A: PPARgamma and the thiazolidinediones: molecular basis for a treatment of 'Syndrome X'? Diabetes Obes Metab 2002, 4:239-248.
54. Knouff C, Auwerx J: Peroxisome proliferator-activated receptor- gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev 2004, 25:899-918.
55. Oberfield JL, Collins JL, Holmes CP, Goreham DM, Cooper JP, Cobb JE, Lenhard JM, Hull-Ryde EA, Mohr CP, Blanchard SG, Parks DJ, Moore LB, Lehmann JM, Plunket K, Miller AB, Milburn MV, Kliewer SA, Willson TM: A peroxisome proliferator-activated receptor gamma ligand inhibits adipocyte differentiation. Proc Natl Acad Sci U S A 1999, 96:6102-6106.
56. Mukherjee R, Hoener PA, Jow L, Bilakovics J, Klausing K, Mais DE, Faulkner A, Croston GE, Paterniti JR Jr: A selective peroxisome proliferator-activated receptor-gamma (PPARgamma) modulator blocks adipocyte differentiation but stimulates glucose uptake in 3T3-L1 adipocytes. Mol Endocrinol 2000, 14:1425-1433.
57. Camp HS, Chaudhry A, Leff T: A novel potent antagonist of peroxisome proliferator-activated receptor gamma blocks adipocyte differentiation but does not revert the phenotype of terminally differentiated adipocytes. Endocrinology 2001, 142:3207-3213.
58. Lee G, Elwood F, McNally J, Weiszmann J, Lindstrom M, Amaral K, Nakamura M, Miao S, Cao P, Learned RM, Chen JL, Li Y: T007 a selective ligand for peroxisome proliferator-activated receptor gamma, functions as an antagonist of biochemical and cellular activities. J Biol Chem 0907, 277:19649-19657.
59. Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, Davis RG, Hull-Ryde EA, Lenhard JM, Patel L, Plunket KD, Shenk JL, Stimmel JB, Therapontos C, Willson TM, Blanchard SG: Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 2002, 28:6640-6650.
60. Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV: Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell 1999, 3:397-403.
61. Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, Soo Kim S, Ha J: The regulation of AMP-activated protein kinase by H(2)O(2). Biochem Biophys Res Commun 2001, 287:92-97.
62. Souza SC, Palmer HJ, Kang YH, Yamamoto MT, Muliro KV, Paulson KE, Greenberg AS: TNF-alpha induction of lipolysis is mediated through activation of the extracellular signal related kinase pathway in 3T3-L1 adipocytes. J Cell Biochem 2003, 89:1077-1086.
63. Floyd ZE, Stephens JM: Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem 2002, 277:4062-4068.
64. Huang WC, Chio CC, Chi KH, Wu HM, Lin WW: Superoxide anion-dependent Raf/MEK/ERK activation by peroxisome proliferator activated receptor gamma agonists 15-deoxydelta( 12,14)-prostaglandin J(2), ciglitazone, and GW1929. Exp Cell Res 2002, 277:192-200.
65. Hedvat M, Jain A, Carson DA, Leoni LM, Huang G, Holden S, Lu D, Corr M, Fox W, Agus DB: Inhibition of HER-kinase activation prevents ERK-mediated degradation of PPARgamma. Cancer Cell 2004, 5:565-574.
66. Tanabe Y, Nakayama K: Mechanical stretching inhibits adipocyte differentiation of 3T3-L1 cells: the molecular mechanism and pharmacological regulation. Nippon Yakurigaku Zasshi 2004, 124:337-344.
67. Tanabe Y, Koga M, Saito M, Matsunaga Y, Nakayama K: Inhibition of adipocyte differentiation by mechanical stretching through ERK-mediated downregulation of PPARgamma2. J Cell Sci 2004, 117:3605-3614.
68. Goransson O, Ryden M, Nilsson R, Arner P, Degerman E: Dimethylaminopurine inhibits metabolic effects of insulin in primary adipocytes. In J Nutr Biochem 2004:303-312.
69. Thupari JN, Landree LE, Ronnett GV, Kuhajda FP: C75 increases peripheral energy utilization and fatty acid oxidation in dietinduced obesity. Proc Natl Acad Sci U S A 2002, 99:9498-9502.
70. St-Onge MP: Relationship between body composition changes and changes in physical function and metabolic risk factors in aging. Curr Opin Clin Nutr Metab Care 2005, 8:523-528.
71. St-Onge MP, Janssen I, Heymsfield SB: Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care 2004, 27:2222-2228.
Last edited:
- Joined
- Jul 25, 2008
- Messages
- 1,700
Fish Oil (DHA) increases metabolism (fat loss)
I thought you might be interested in this because almost no nutritionists or people involved in bodybuilding know this.
But in the world of aging and longevity research there is the knowledge that the lipid make up of the cellular membrane influences cellular metabolism. High DHA content in phospholipids of the cellular membrane is associated with high metabolic activity and this leads into the "membrane pacemaker" theory of metabolism.
This theory proposes that highly polyunsaturated acyl chains impart physical properties to cellular membrane bilayers that enhance and speed up the molecular activity of membrane proteins and consequently the metabolic activity of cells, tissues and the whole animal.
There is a positive correlation in the animal kingdom between body size and cellular metabolism with smaller animals possessing cellular membranes with higher DHA content and thus higher cellular metabolisms. Larger animals, humans for instance have cellular membranes with a lot less DHA and thus slower cellular metabolism.
All of this is correlated to lifespan. Highly polyunsaturated acyl chains (primarily DHA) are very susceptible to peroxidative damage. This kind of damage shortens lifespan.
The brain however is not correlated to any of this and in humans posses high DHA content in the cellular membrane & thus higher metabolism. It is thought that evolution probably weeded out those sluggish thought creatures that had slower brain cell metabolism.
How does it work? Well the physical properties of polyunsaturates primarily DHA are such that these lipid chains are flexible and active compared to unsaturated lipids. Because of the active or rapid movement of DHA lipids they exert lateral pressure on neighboring molecules in the cellular membrane. This creates greater activity in membrane enzymes, Na+/K+-ATPase molecules and thus ion channels become approximtely 25% more active. A large part of cellular energy goes into operating those channels.
Similar sorts of activity occur in the mitochondrial membrane proteins.
A good & recent review of all of this is The links between membrane composition, metabolic rate and lifespan, A.J. Hulbert, Comparative Biochemistry and Physiology, Part A 150 (2008) 196–203
Now we know from source material such as Evolutionary Aspects of Diet, the Omega-6/Omega-3 Ratio, and Gene Expression by Artemis P. Simopoulos found in the book Phytochemicals: Nutrient-Gene Interactions, Mark S. Meskin (Editor), CRC; 1 edition (February 22, Phytochemicals: Nutrient-Gene Interactions, Mark S. Meskin (Editor), CRC; 1 edition (February 22, 2006) that:
So if you ingest a large quantity of Omega 3 fatty acids which contain high DHA content you will alter the makeup of the cellular membrane such that it is composed of more DHA which will increase cellular metabolism.
This is great for dieting but increases the potential oxidative damage. Enough to effective lifespan? Probably not. But it should increase energy expenditure and thus be beneficial on a diet.
I thought you might be interested in this because almost no nutritionists or people involved in bodybuilding know this.
But in the world of aging and longevity research there is the knowledge that the lipid make up of the cellular membrane influences cellular metabolism. High DHA content in phospholipids of the cellular membrane is associated with high metabolic activity and this leads into the "membrane pacemaker" theory of metabolism.
This theory proposes that highly polyunsaturated acyl chains impart physical properties to cellular membrane bilayers that enhance and speed up the molecular activity of membrane proteins and consequently the metabolic activity of cells, tissues and the whole animal.
There is a positive correlation in the animal kingdom between body size and cellular metabolism with smaller animals possessing cellular membranes with higher DHA content and thus higher cellular metabolisms. Larger animals, humans for instance have cellular membranes with a lot less DHA and thus slower cellular metabolism.
All of this is correlated to lifespan. Highly polyunsaturated acyl chains (primarily DHA) are very susceptible to peroxidative damage. This kind of damage shortens lifespan.
The brain however is not correlated to any of this and in humans posses high DHA content in the cellular membrane & thus higher metabolism. It is thought that evolution probably weeded out those sluggish thought creatures that had slower brain cell metabolism.
How does it work? Well the physical properties of polyunsaturates primarily DHA are such that these lipid chains are flexible and active compared to unsaturated lipids. Because of the active or rapid movement of DHA lipids they exert lateral pressure on neighboring molecules in the cellular membrane. This creates greater activity in membrane enzymes, Na+/K+-ATPase molecules and thus ion channels become approximtely 25% more active. A large part of cellular energy goes into operating those channels.
Similar sorts of activity occur in the mitochondrial membrane proteins.
A good & recent review of all of this is The links between membrane composition, metabolic rate and lifespan, A.J. Hulbert, Comparative Biochemistry and Physiology, Part A 150 (2008) 196–203
Now we know from source material such as Evolutionary Aspects of Diet, the Omega-6/Omega-3 Ratio, and Gene Expression by Artemis P. Simopoulos found in the book Phytochemicals: Nutrient-Gene Interactions, Mark S. Meskin (Editor), CRC; 1 edition (February 22, Phytochemicals: Nutrient-Gene Interactions, Mark S. Meskin (Editor), CRC; 1 edition (February 22, 2006) that:
"... the polyunsaturated fatty acid (PUFA) composition of cell membranes is to a great extent dependent on the dietary intake."
So if you ingest a large quantity of Omega 3 fatty acids which contain high DHA content you will alter the makeup of the cellular membrane such that it is composed of more DHA which will increase cellular metabolism.
This is great for dieting but increases the potential oxidative damage. Enough to effective lifespan? Probably not. But it should increase energy expenditure and thus be beneficial on a diet.
Similar threads
Popular tags
aas
aas testing
anabolic steroids
anabolics online
anabolid steroids
anadrol
anadrol drol tabs inj
anavar
anavar and winnie
body building
body building supplements
bodybuilder
bodybuilding
bodybuilding steroid test
clenbuterol
cycle
deca tren dosage
deca-durobolin
dianabol
dianabol and oxy
dragon pharma
gear
hcg
hgh
motivation
muscle building
muscle mass
nandrolone
pct
peptide
peptide suggestions
peptides
steroid cycle
steroids
suspension
sustanon
test
test 400
test cyp
test cypionate
test prop
testosterone
testosterone boosters
testosterone cypionate
testsuspension
tren
tren ace
tren ace buy
trenbolone acetate
winstrol
Popular tags
aas
aas testing
anabolic steroids
anabolics online
anabolid steroids
anadrol
anadrol drol tabs inj
anavar
anavar and winnie
body building
body building supplements
bodybuilder
bodybuilding
bodybuilding steroid test
clenbuterol
cycle
deca tren dosage
deca-durobolin
dianabol
dianabol and oxy
dragon pharma
gear
hcg
hgh
motivation
muscle building
muscle mass
nandrolone
pct
peptide
peptide suggestions
peptides
steroid cycle
steroids
suspension
sustanon
test
test 400
test cyp
test cypionate
test prop
testosterone
testosterone boosters
testosterone cypionate
testsuspension
tren
tren ace
tren ace buy
trenbolone acetate
winstrol
Members online
- rrozell
- aptry
- Klime
- jwalk
- Jman88
- IronMindset
- Blobmarine
- shaker89
- td
- Jliltman
- Massivekaos
- Eternalpain
- Squat to death
- juiceball44
- HalfwayHulk
- skinnyrunt
- chooch
- Powerlifter
- The OC
- buck
- musclemuscle
- Mike73
- goal245
- LATS
- Berst
- hawkmoon
- scooped
- romo
- Tyrone
- Canuck54
- Elvis755
- mr.e01
- Diesel_Dick
- Haggelus
- uL7iMa
- SuperJew
- dsteelo455
- UnitedPeptides
- ChefTren
- tommyguns2
- KrAven
- qbkilla
- Big Vin
- phani
- Thyak
- mbuilder
- Beachwood
- biglizard225
- brokeneck
- BigGame
Total: 1,448 (members: 1,440, guests: 8)
Forum statistics
- Total page views
- 636,848,940
- Threads
- 142,358
- Messages
- 2,950,490
- Members
- 182,513
- Latest member
- vic892











































































